The document discusses severity assessment of adverse drug reactions (ADRs). It describes several scales used to assess the causality and severity of ADRs, including:
- The WHO-UMC Causality Assessment Scale which categorizes ADR causality as certain, probable, possible, unlikely, conditional/unclassified, or unassessable.
- Scales that categorize ADR severity as mild, moderate, severe or lethal based on factors like treatment required and effects on hospitalization.
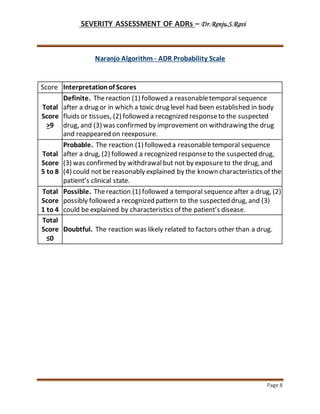
- The Naranjo Algorithm/ADR Probability Scale which assigns a probability score to determine if an ADR is definite, probable, possible, or doubtful based on responses to 10 questions.