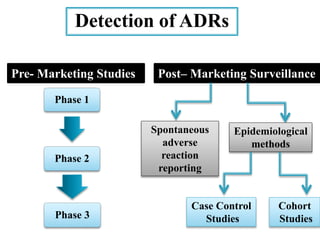
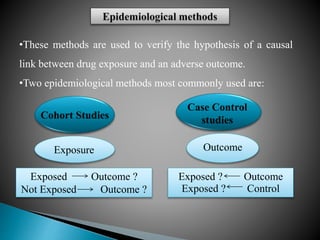
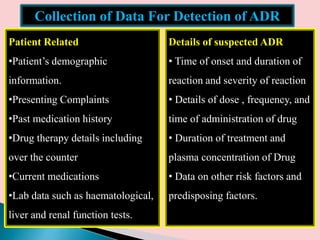
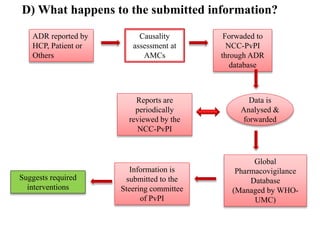
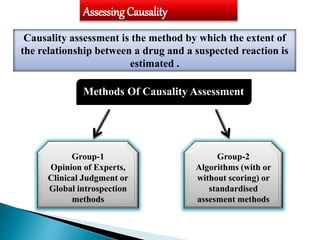
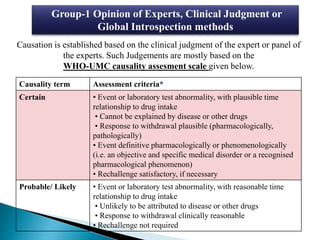
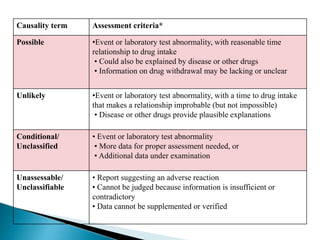
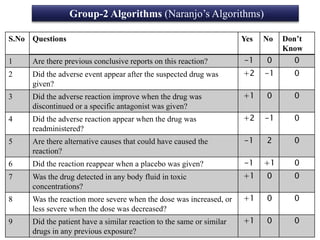
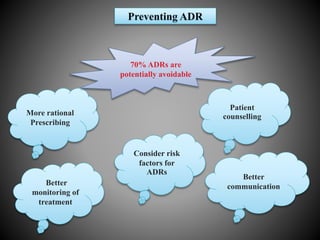
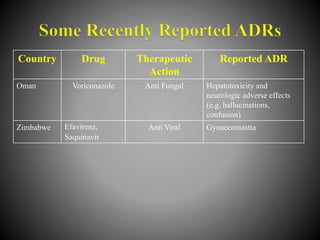
This document discusses adverse drug reactions (ADRs). It defines ADRs and provides various classifications based on type, onset, and severity. It also outlines methods for detecting, reporting, and assessing causality of ADRs. Key points covered include pre-marketing and post-marketing surveillance strategies, the roles of healthcare professionals in detection, and the importance of reporting all suspected ADRs to pharmacovigilance centers. Preventing ADRs requires rational prescribing, risk communication, and close patient monitoring.