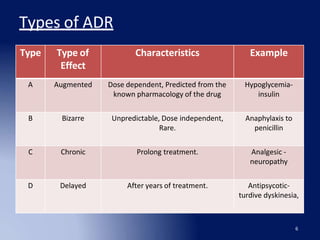
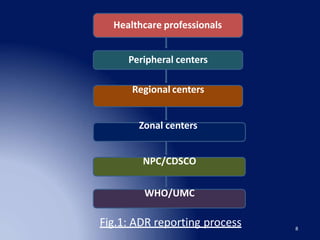
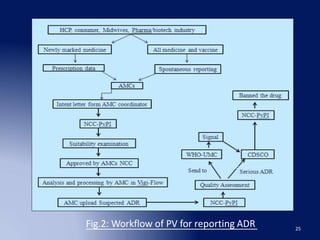
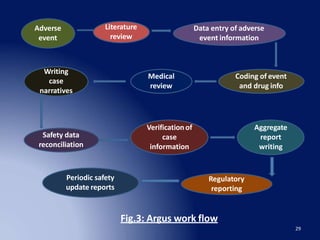
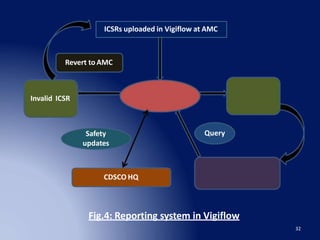
The document discusses adverse drug reaction (ADR) reporting tools. It provides a brief history of pharmacovigilance and describes the ADR reporting process from healthcare professionals to regional and zonal centers, and finally to national and global databases. It emphasizes the importance of post-marketing ADR monitoring to identify uncommon or rare reactions not detected during clinical trials. Key aspects like what information to report, who can report, and how to submit reports using standardized forms are summarized. Common ADR reporting software tools like Argus are also highlighted.