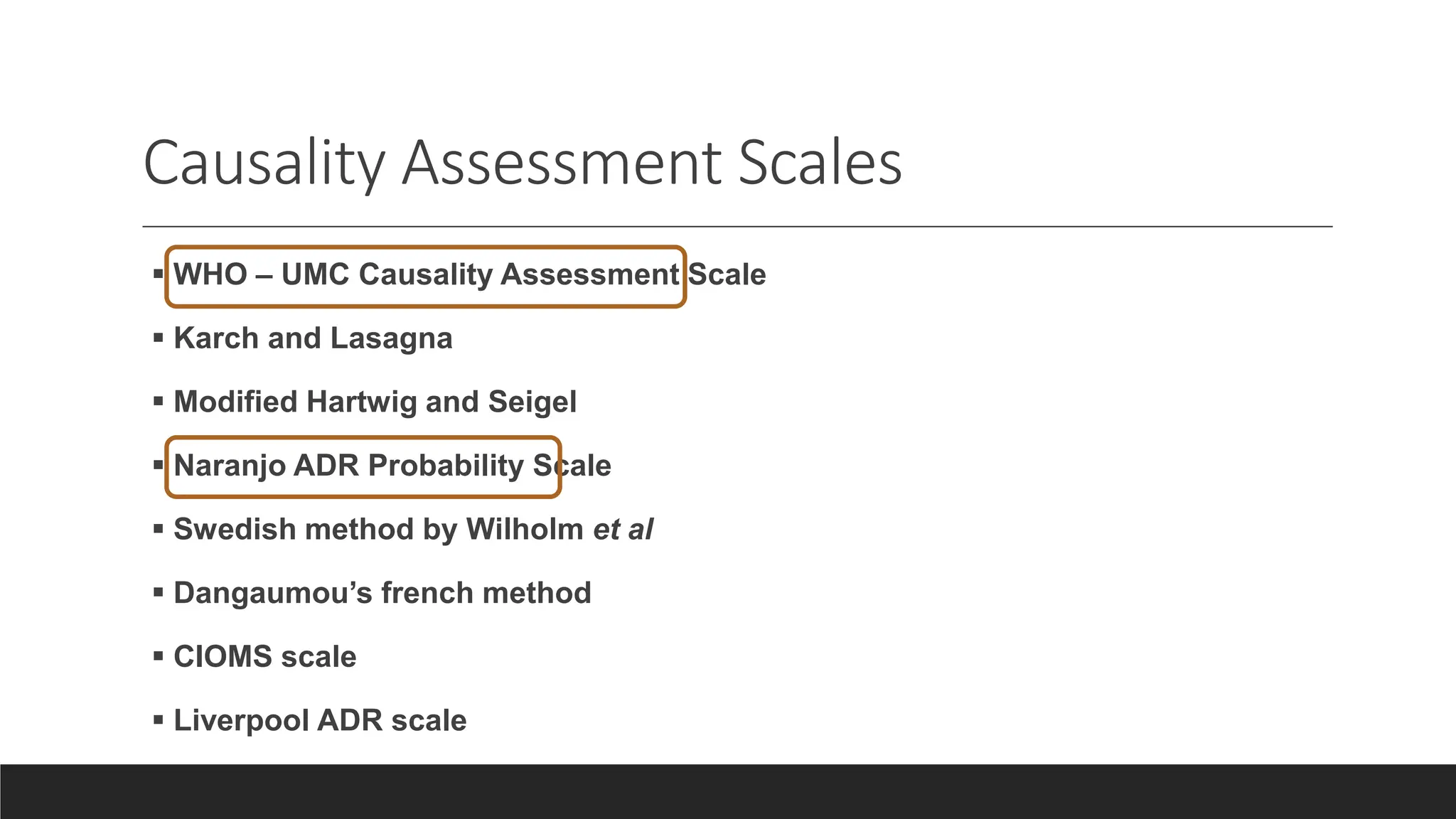
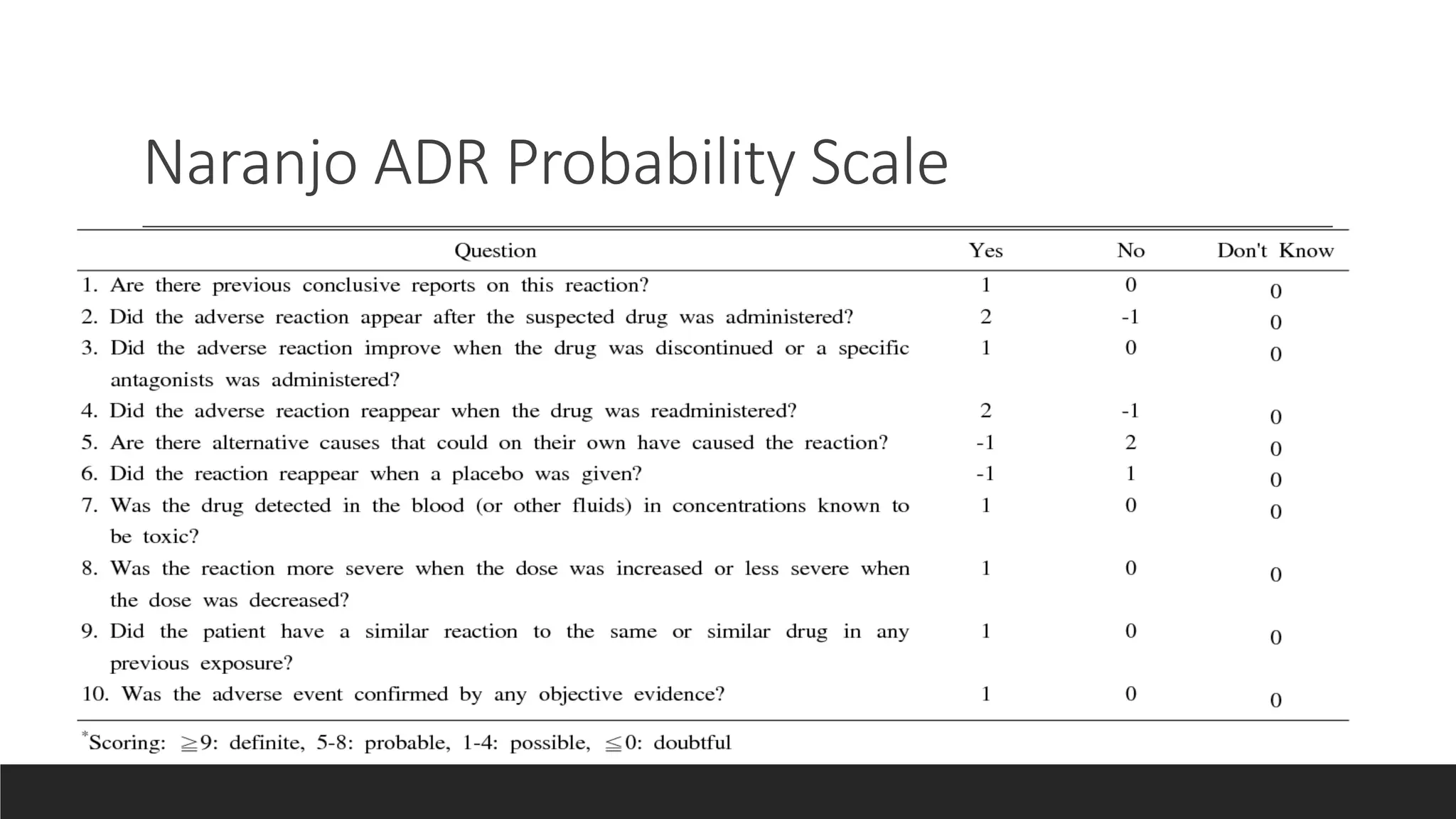
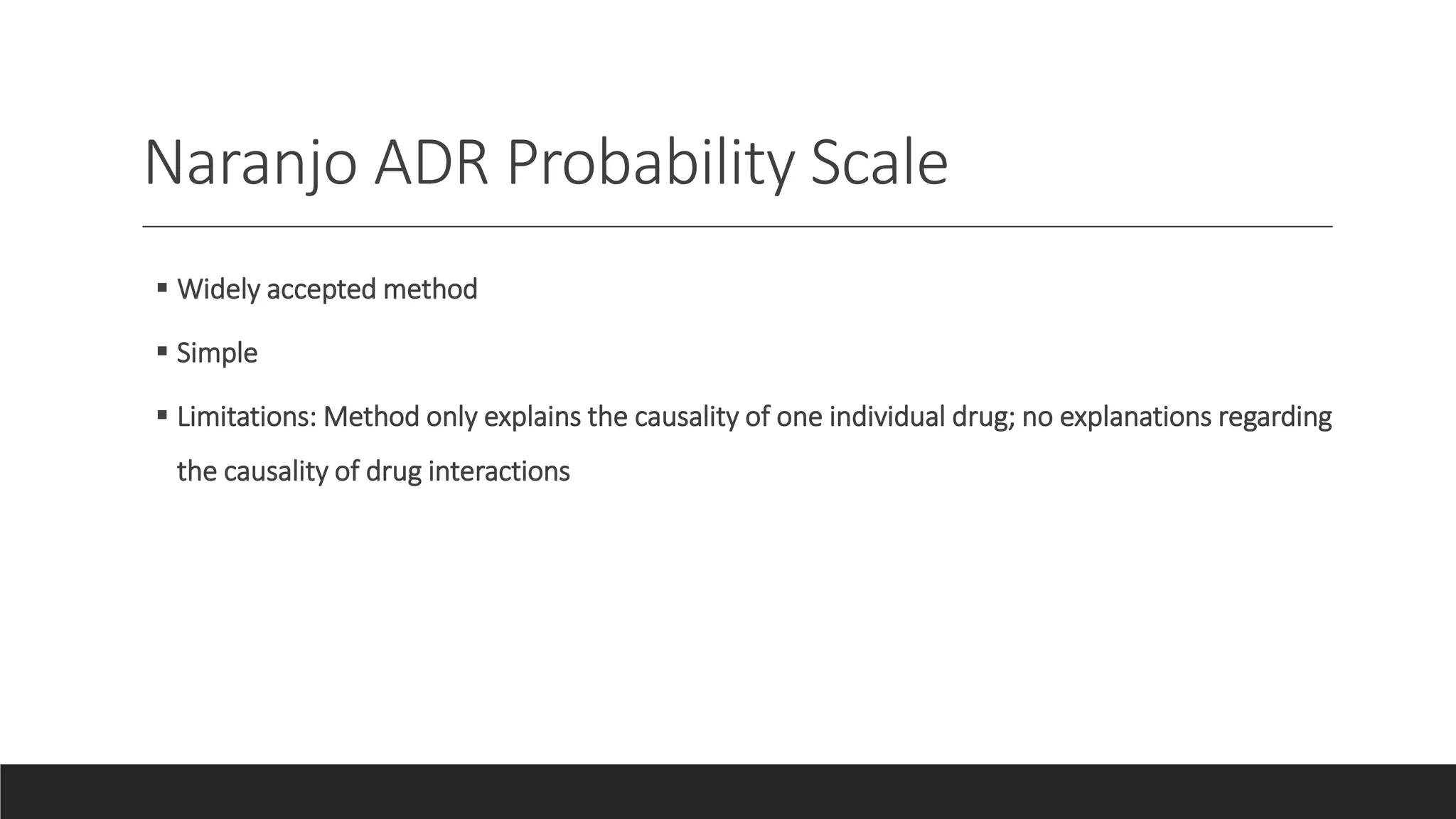
The document discusses adverse drug reactions (ADRs), including definitions, classifications, reporting and management. Some key points:
- ADRs occur in 5-10% of patients annually. An ADR is an unintended reaction to a drug that occurs at normal doses.
- Serious ADRs result in death, hospitalization, disability or birth defects. Suspected ADRs have a reasonable possibility of being caused by a drug.
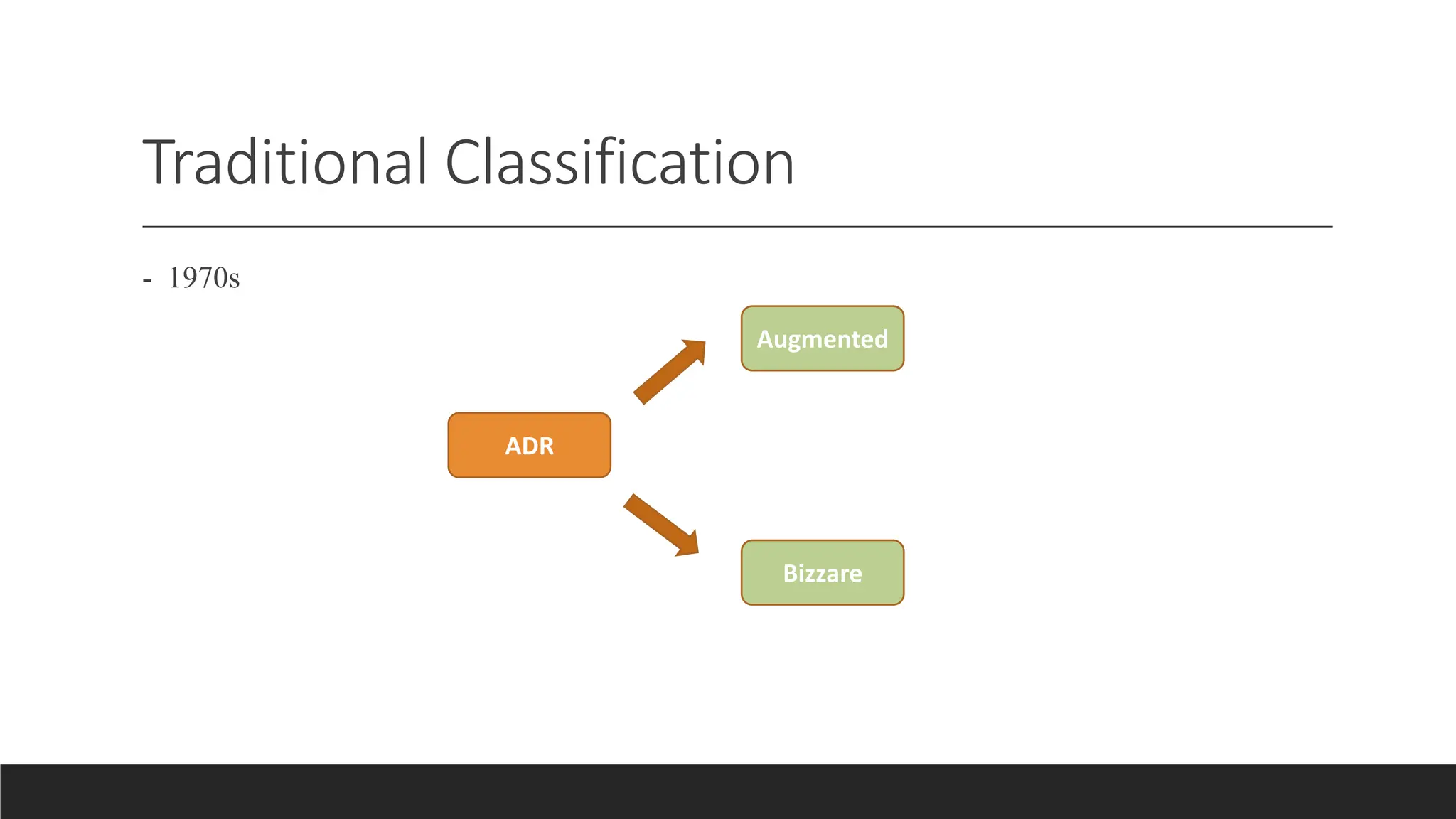
- ADRs can be classified based on dose (augmented, bizarre), time (early, late), or susceptibility (gender, age, genetics).
- Healthcare providers should report all suspected ADRs to monitoring centers to identify safety issues. Prevention