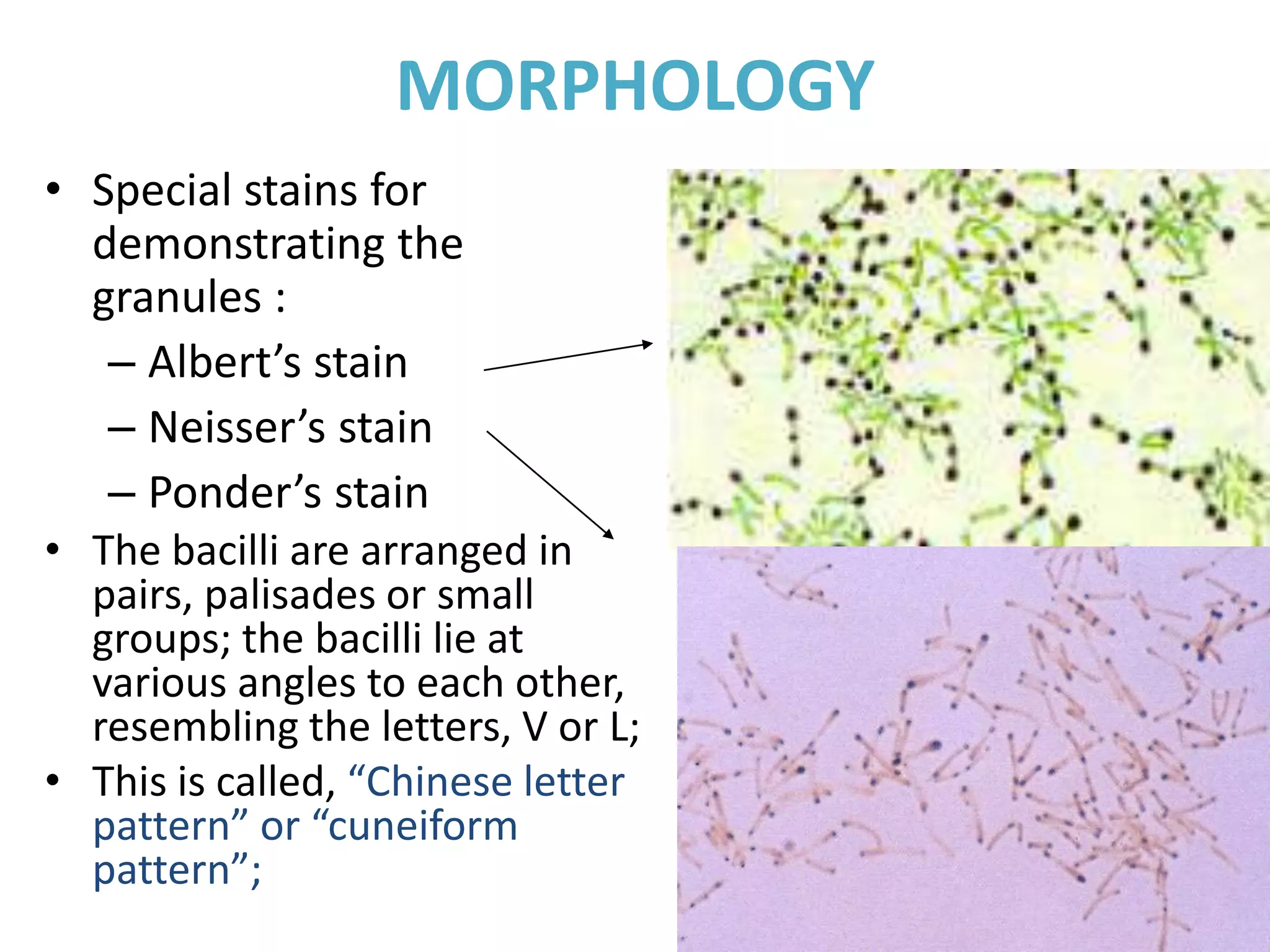
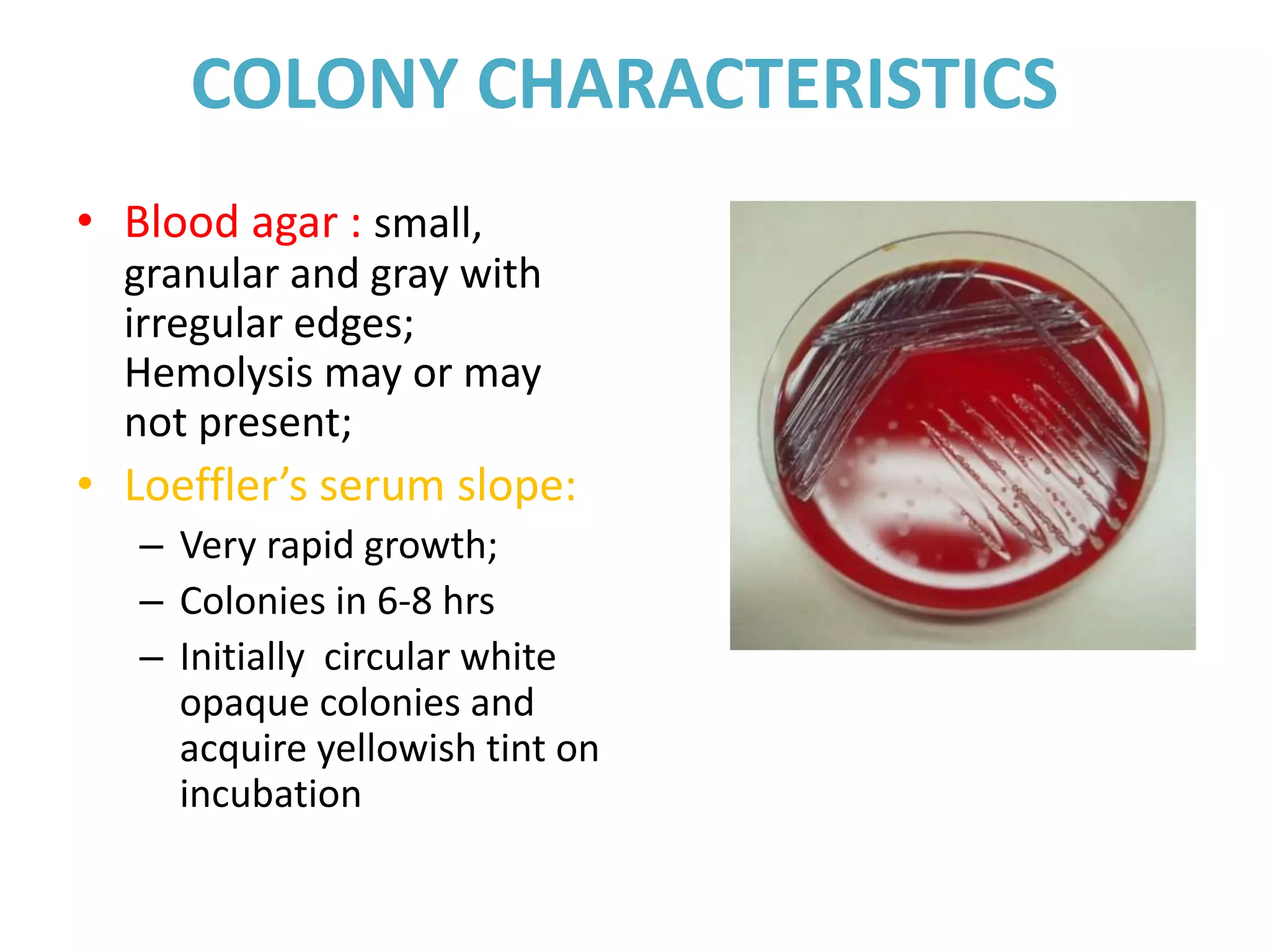
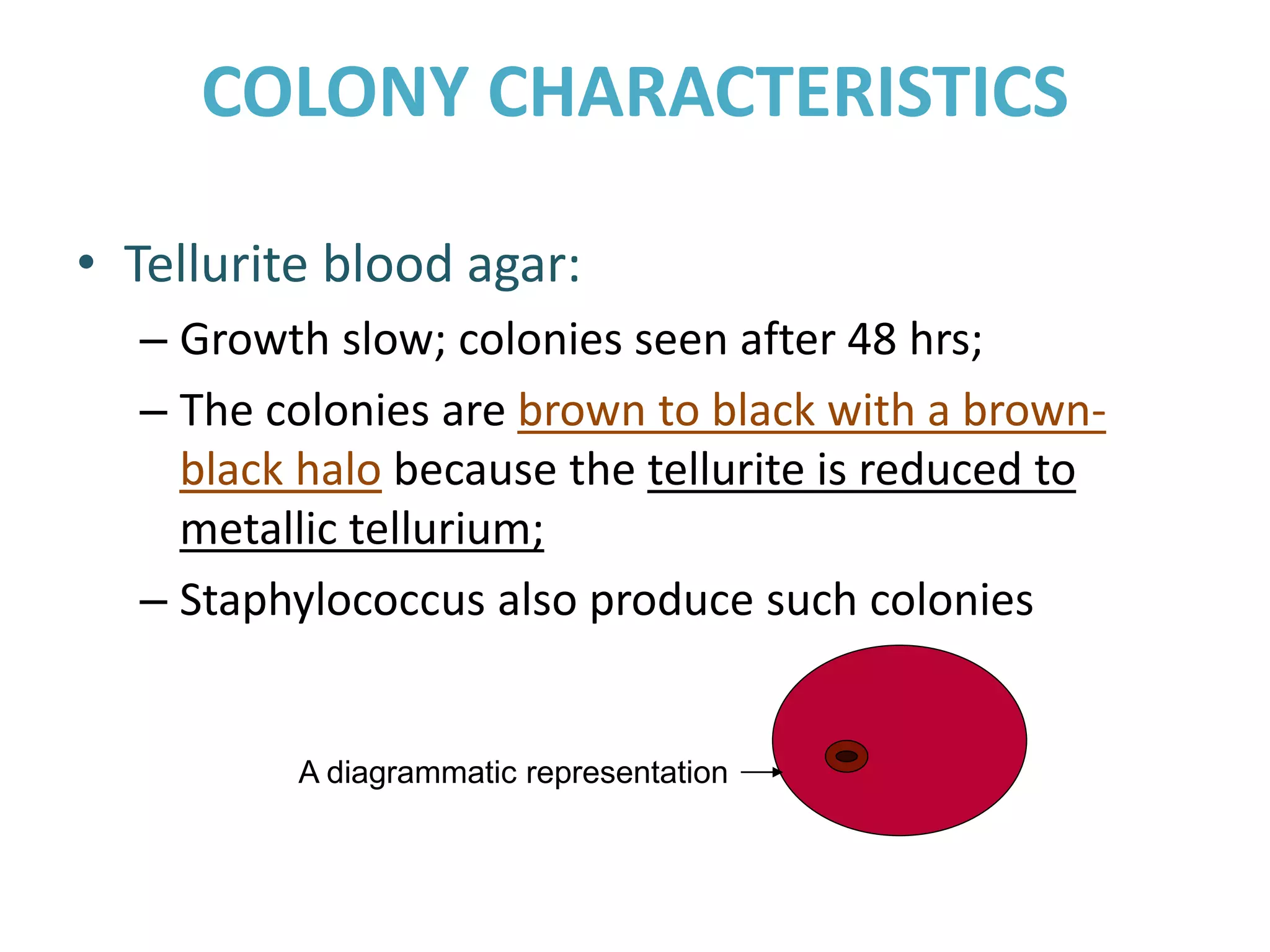
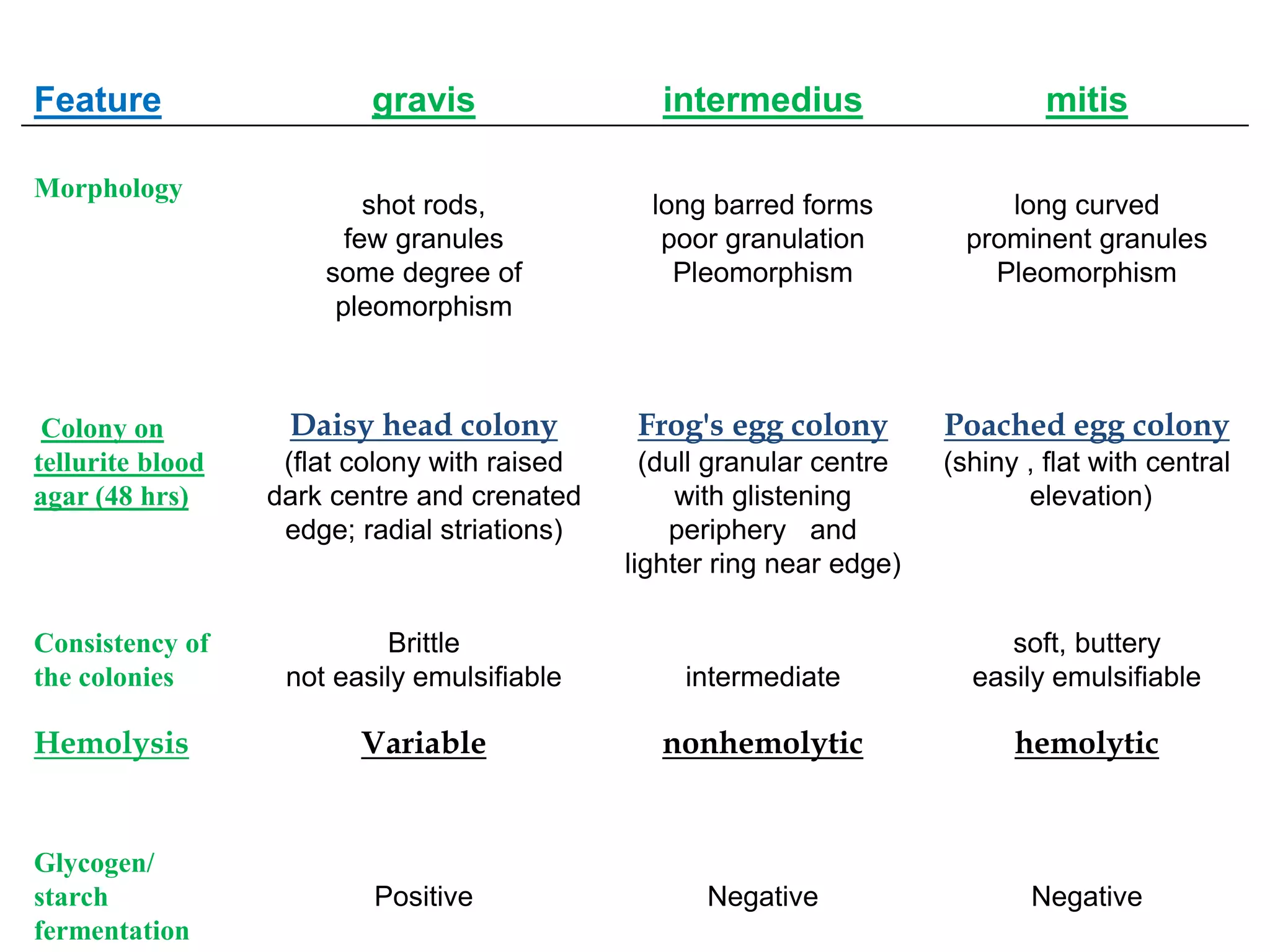
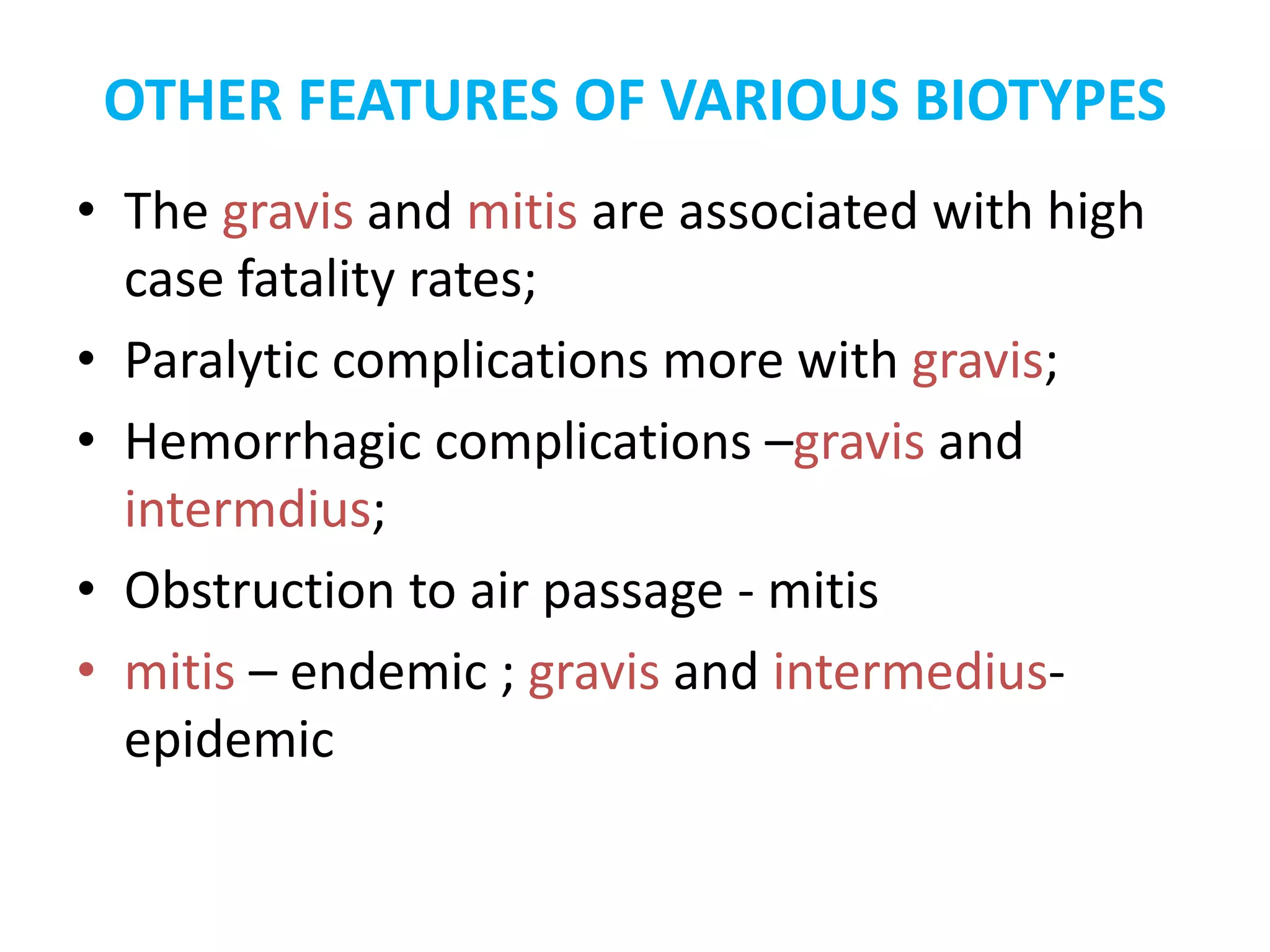
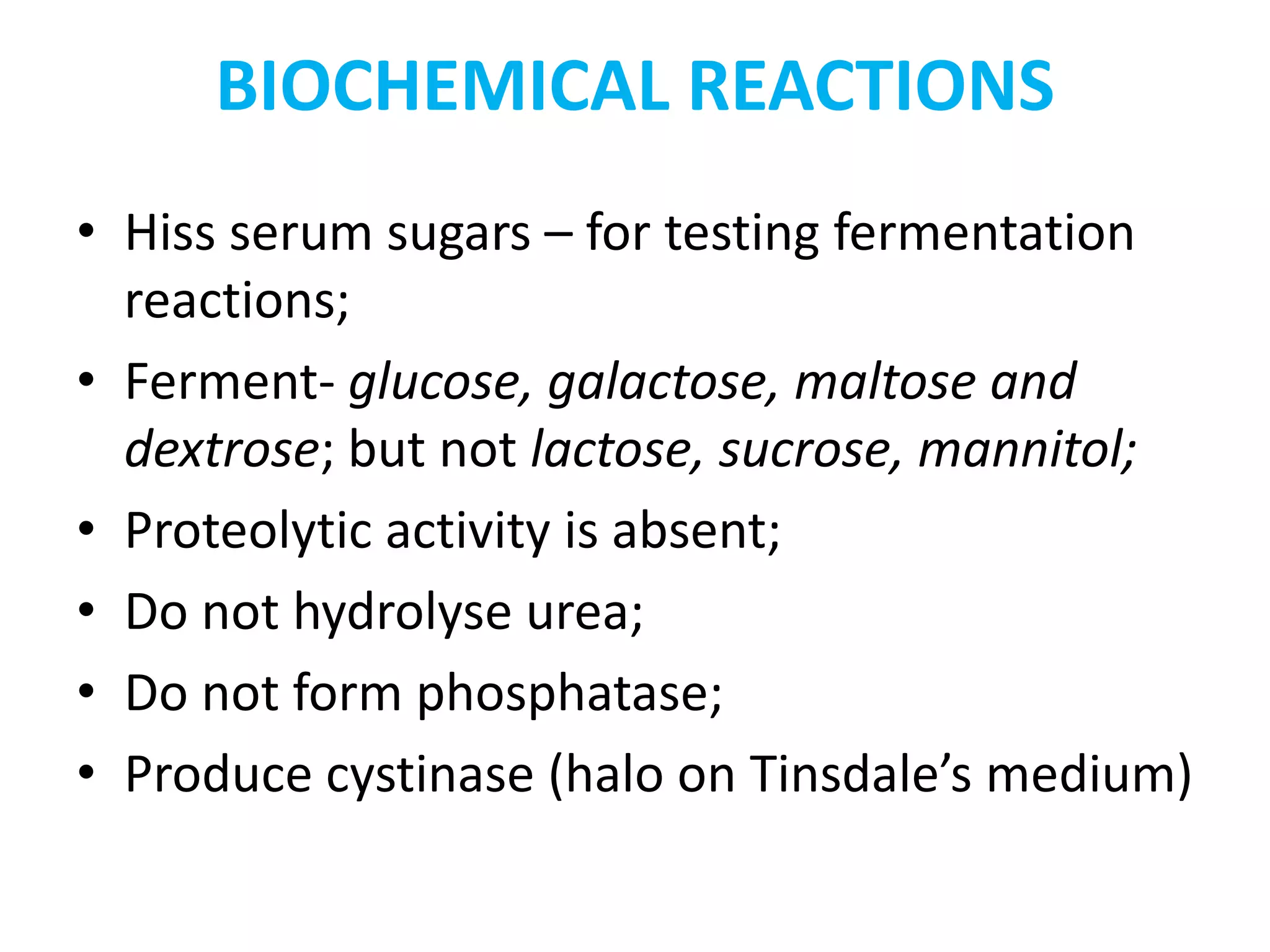
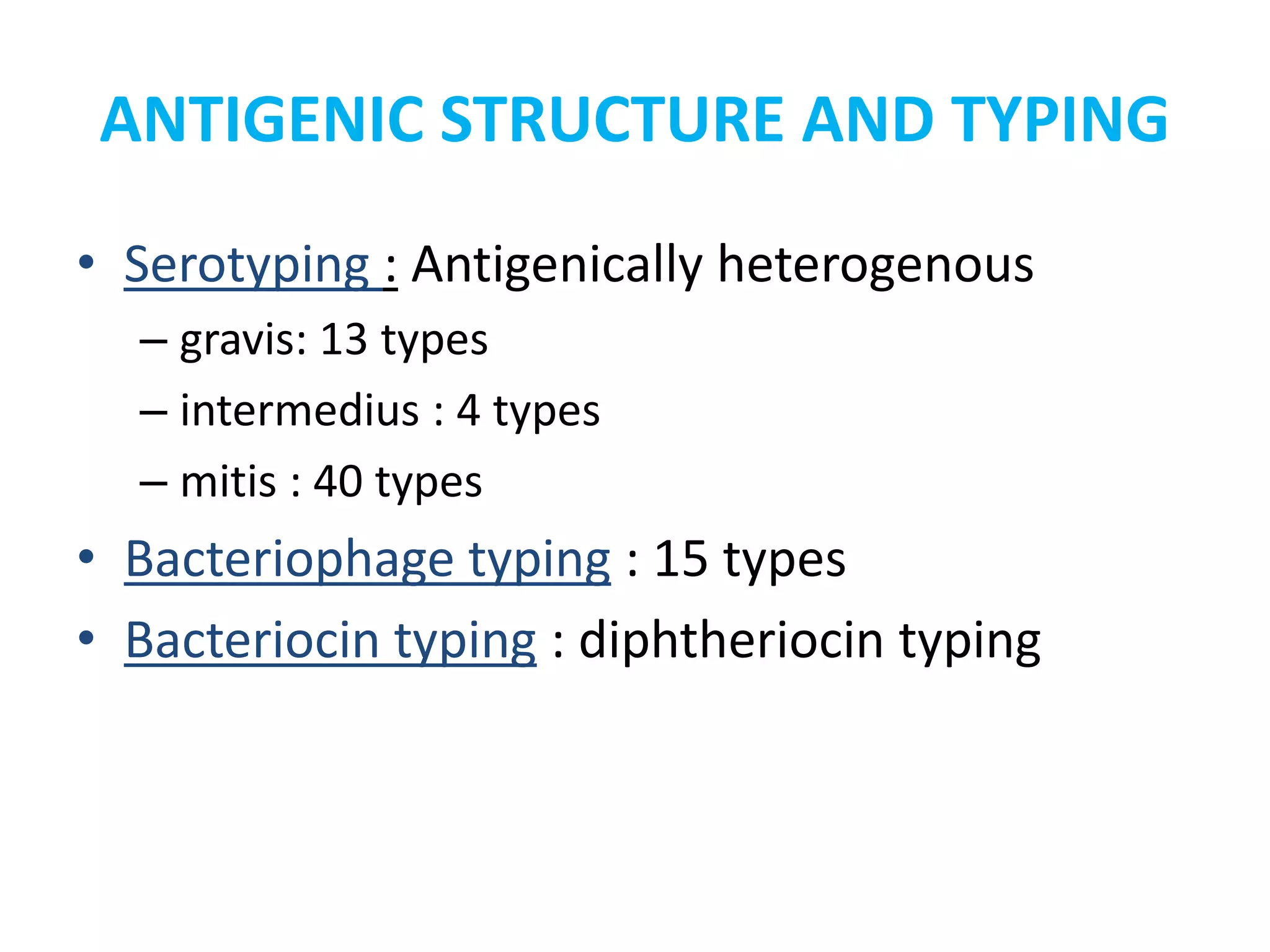
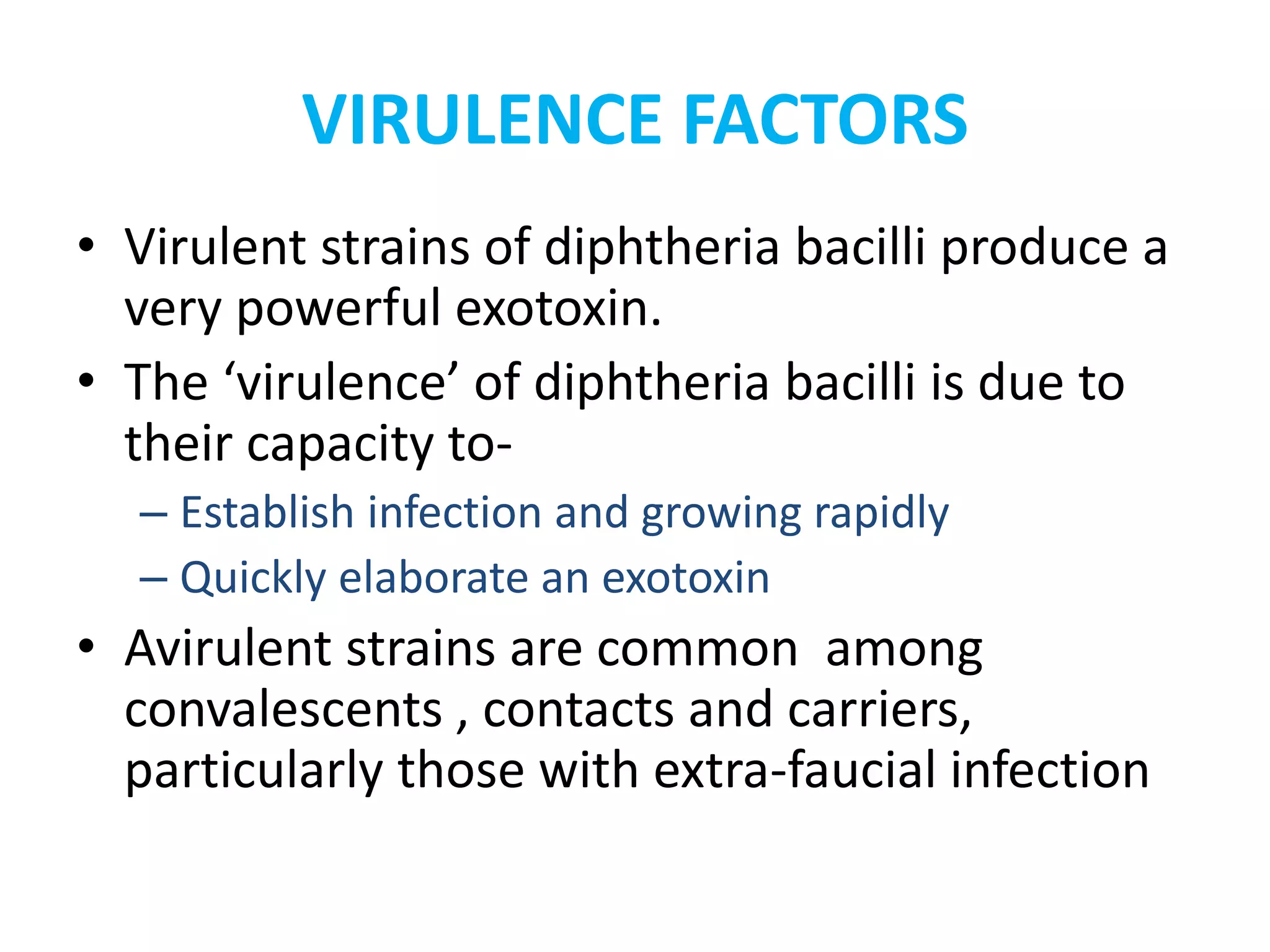
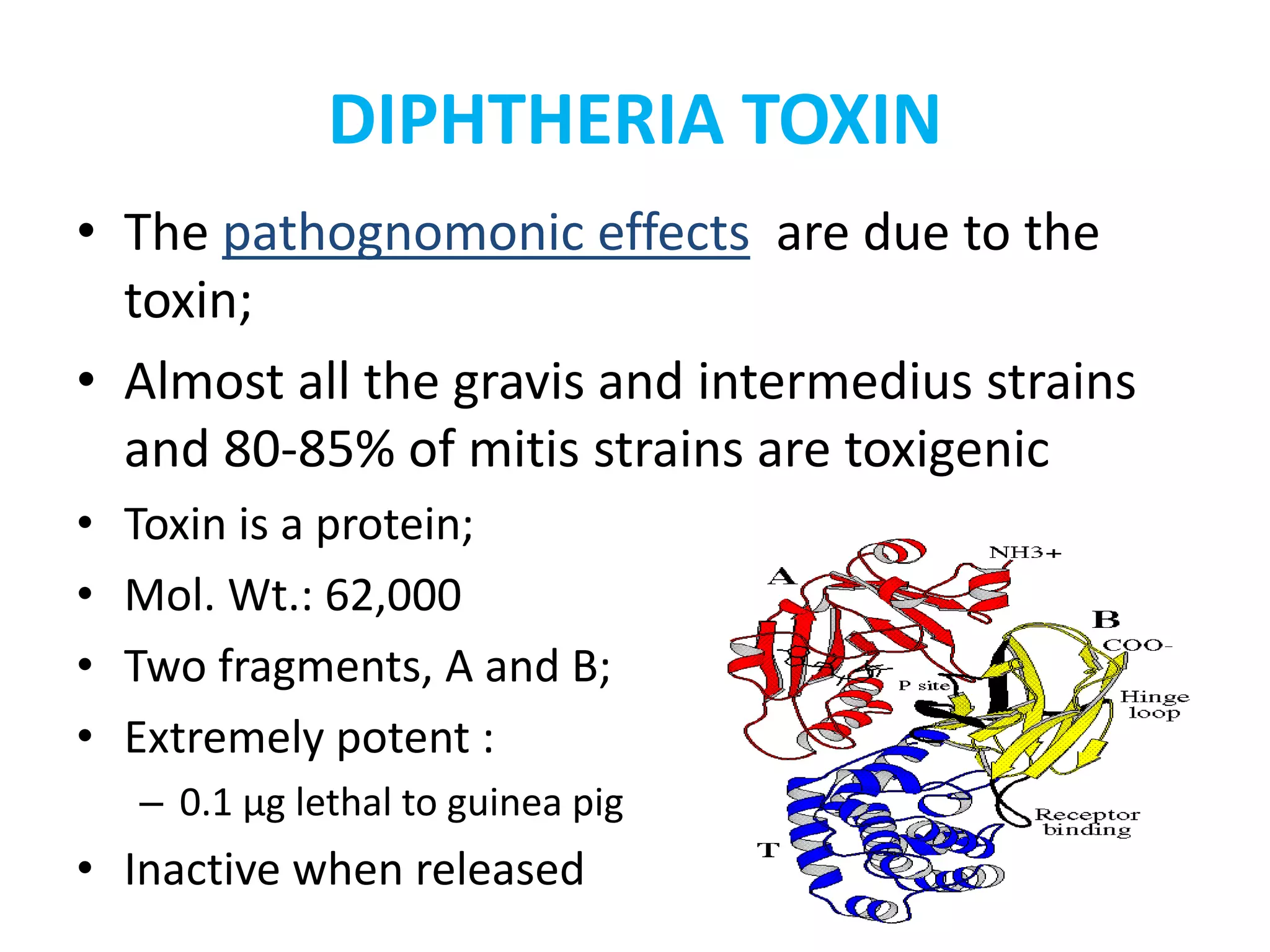
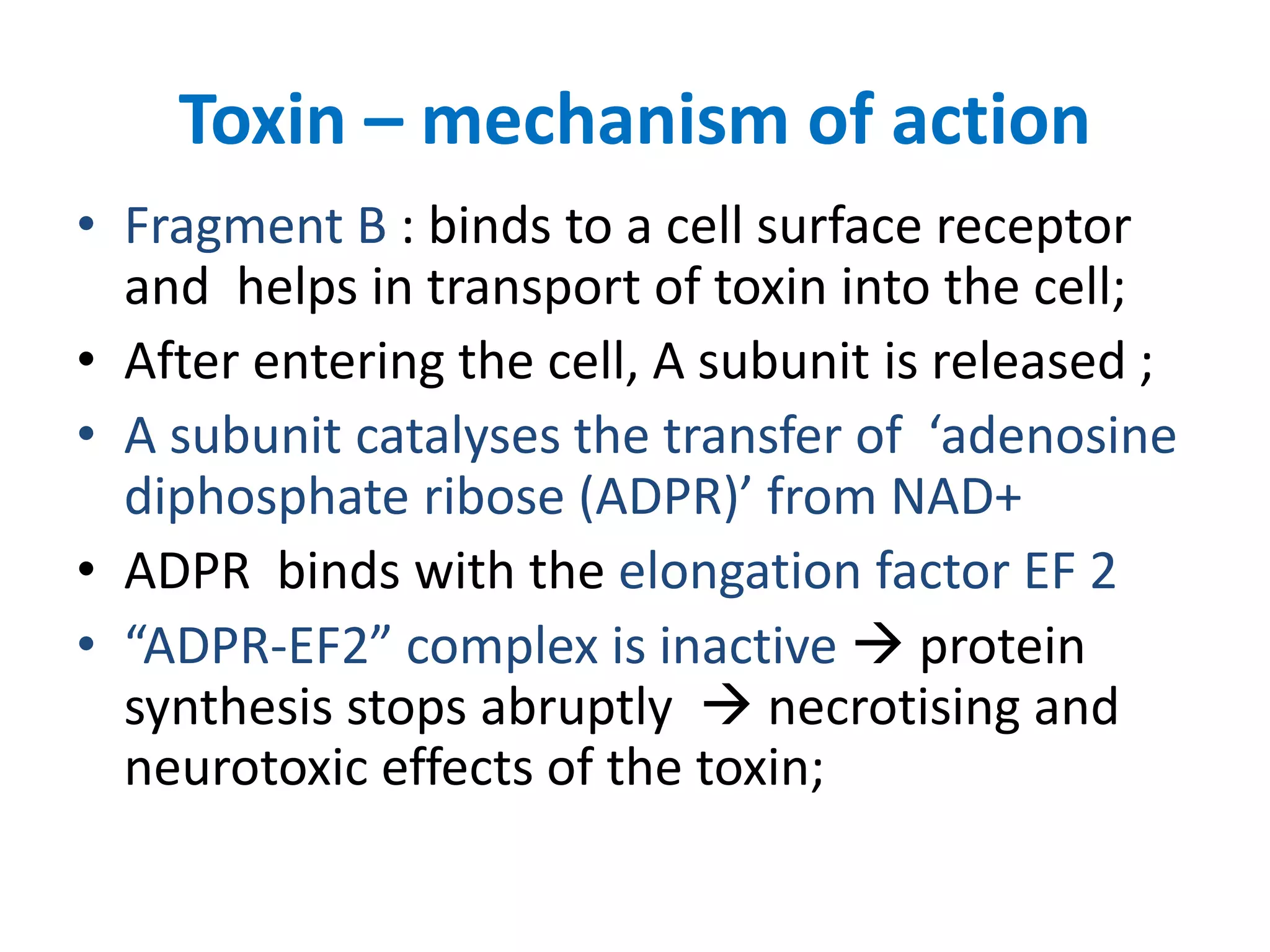
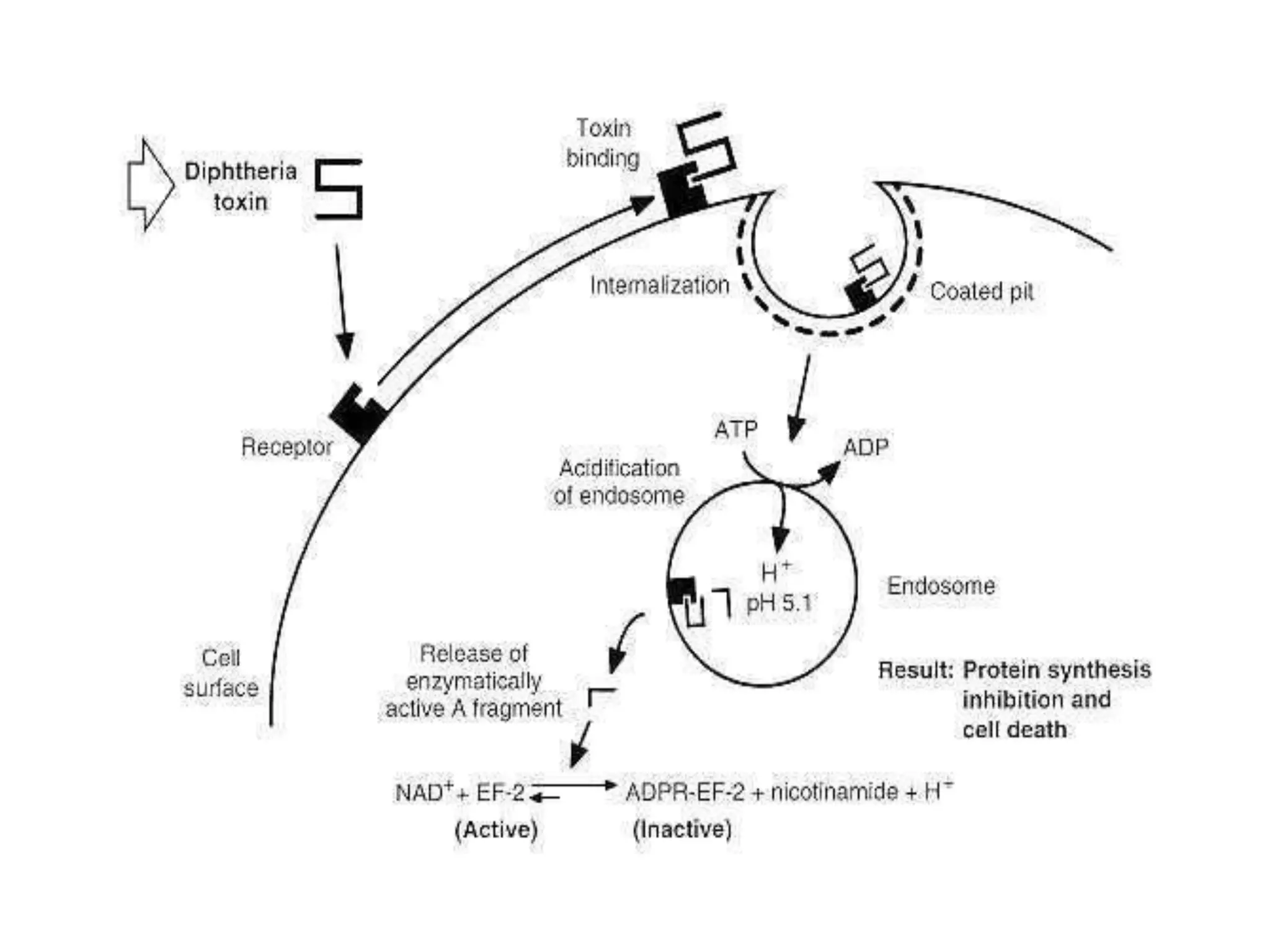
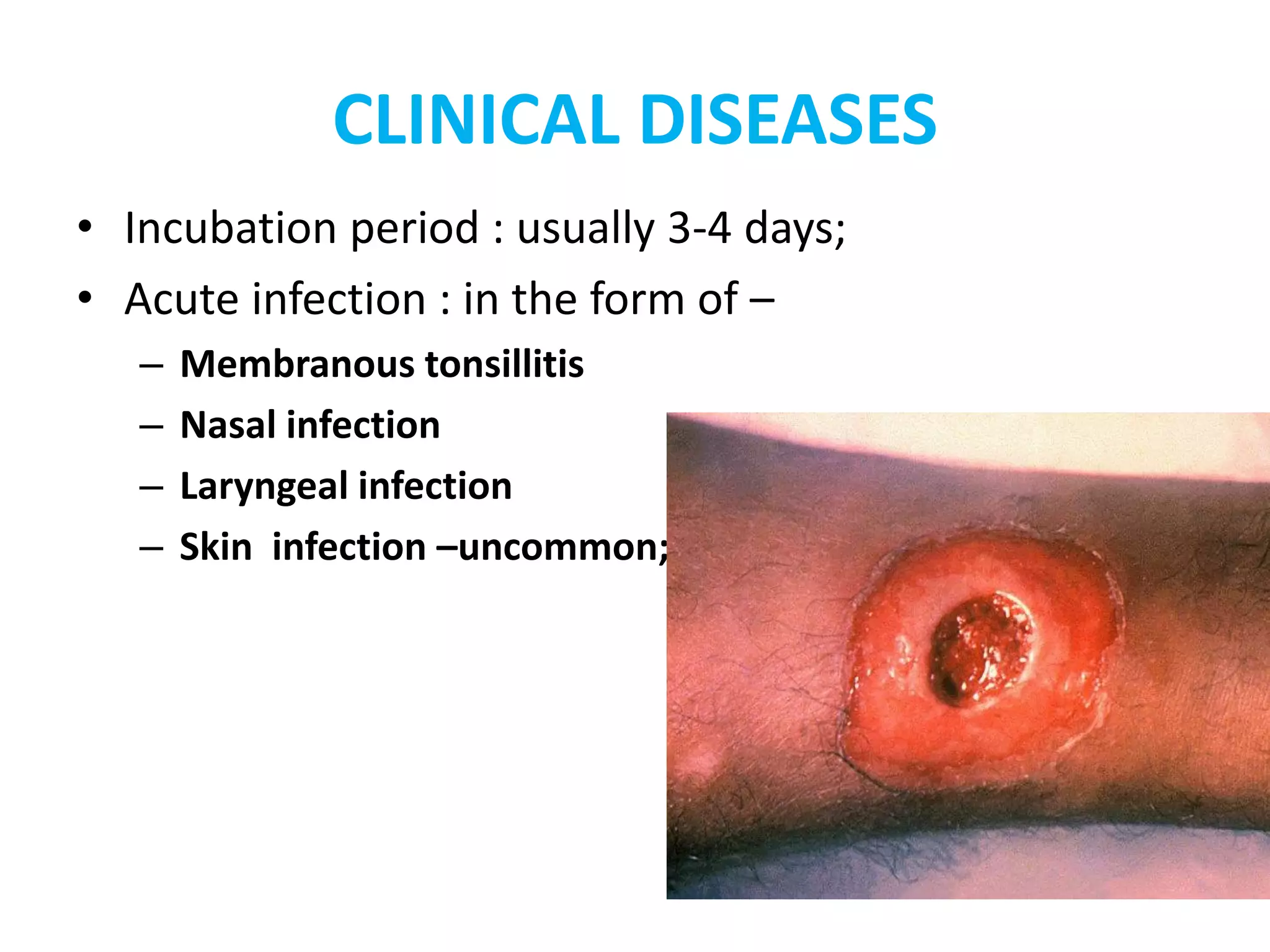
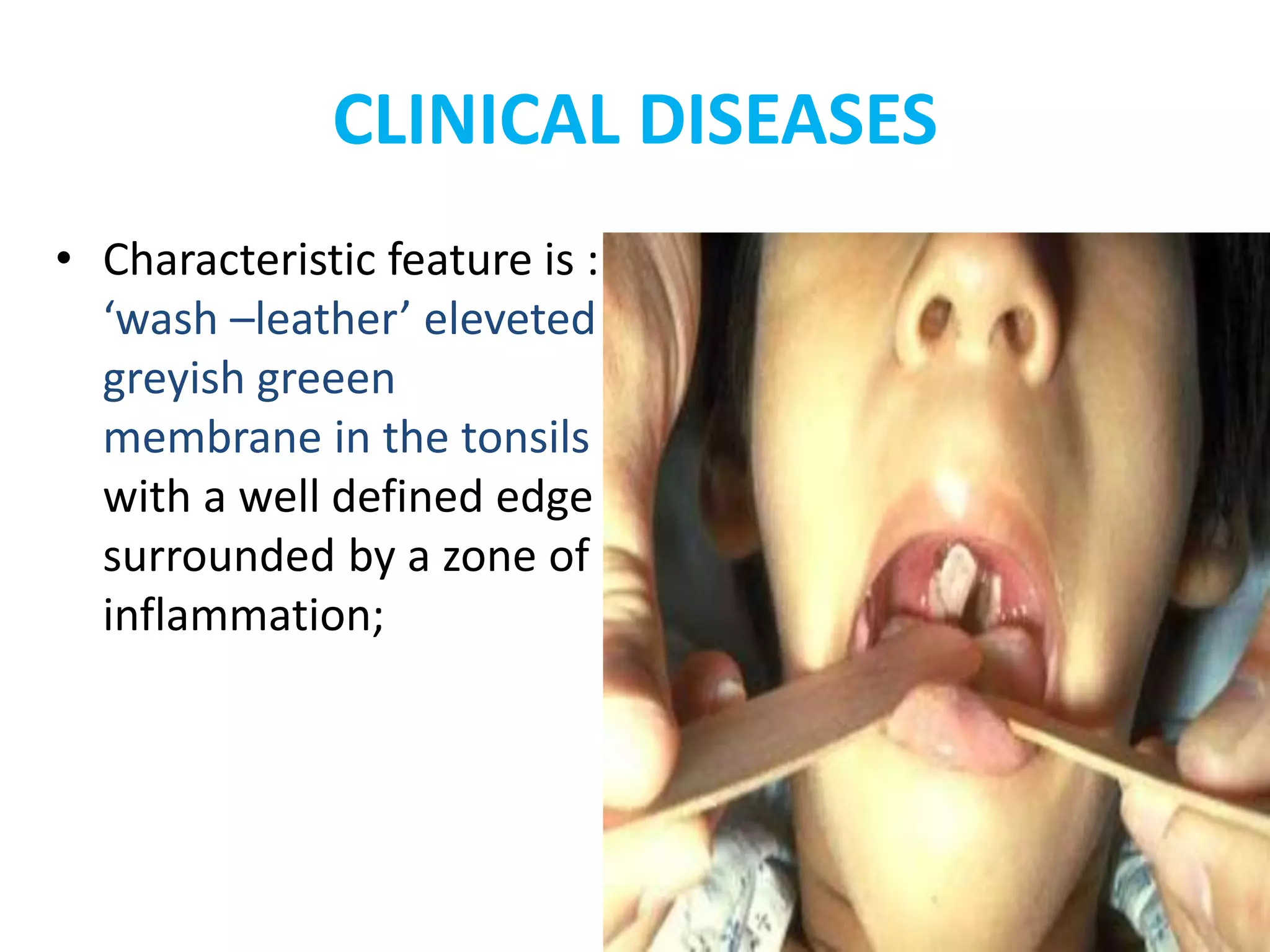
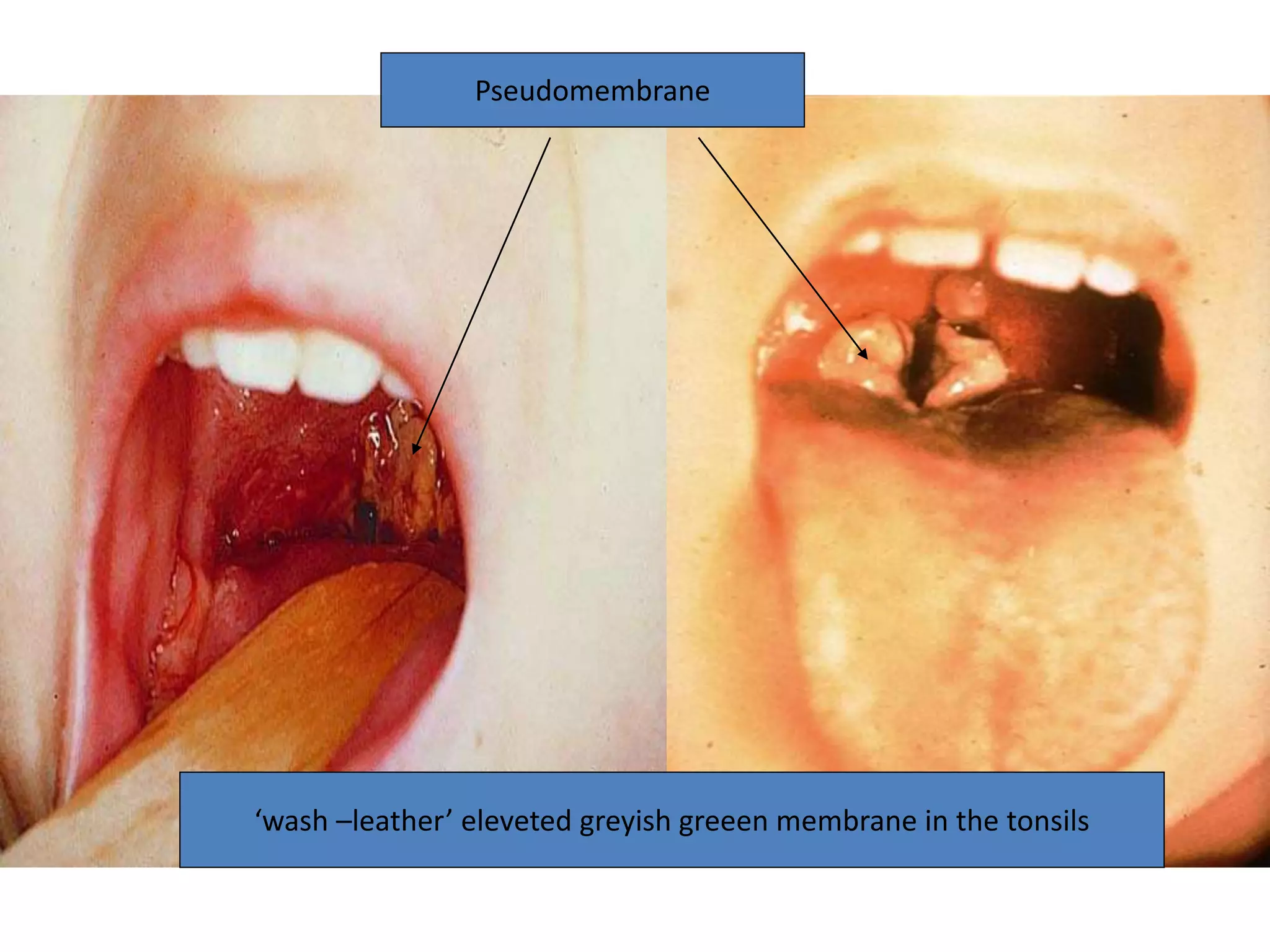
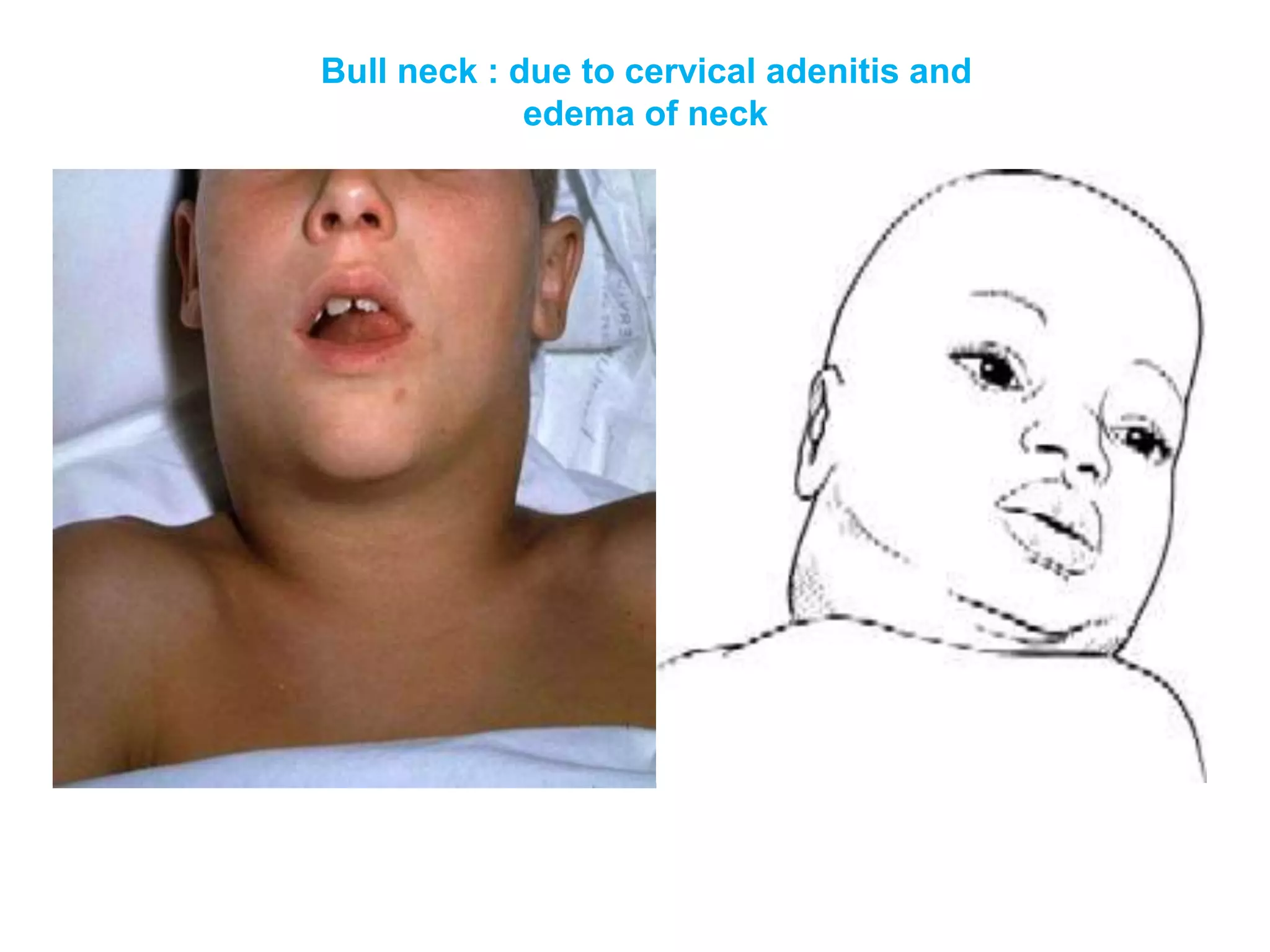
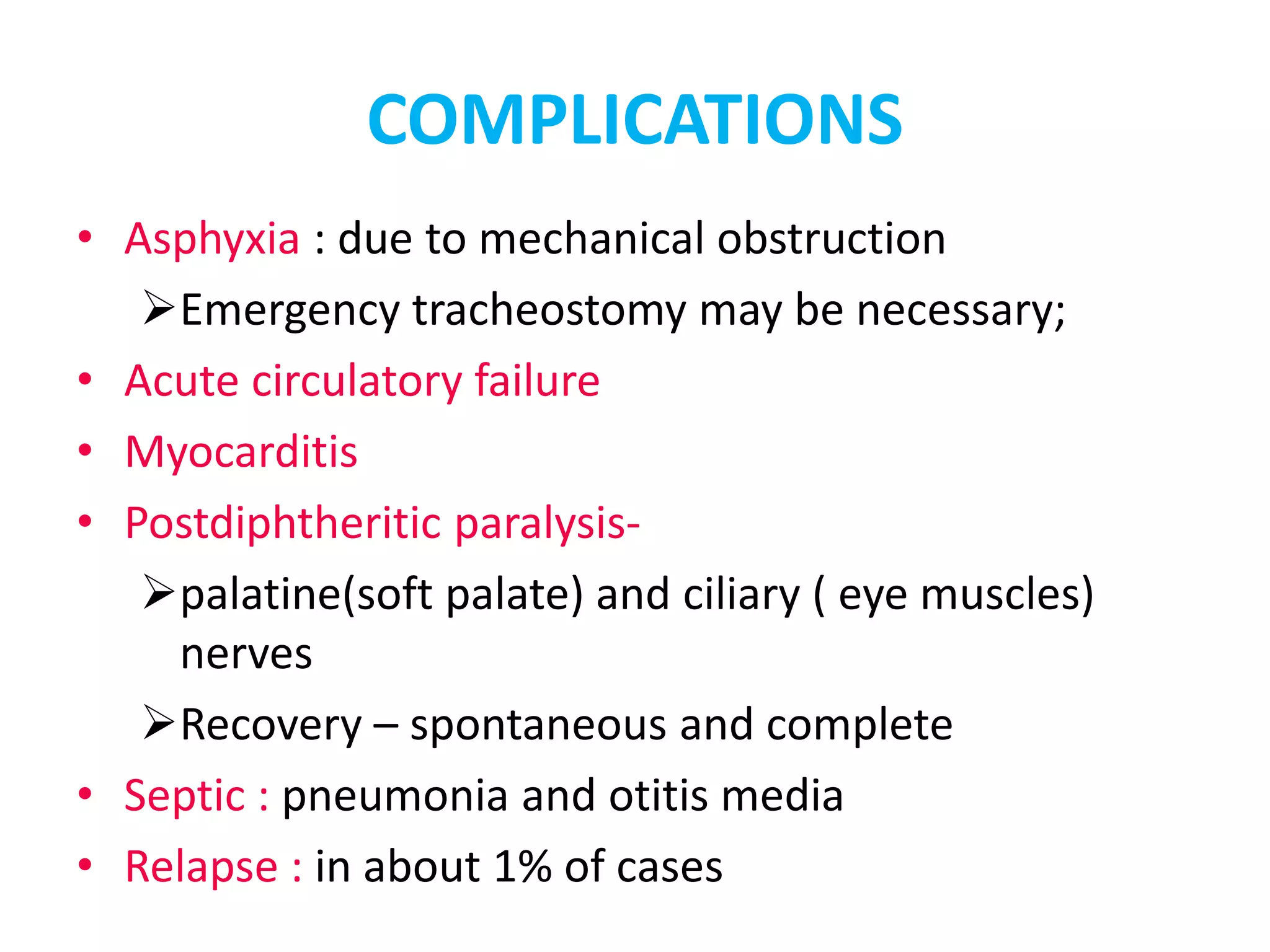
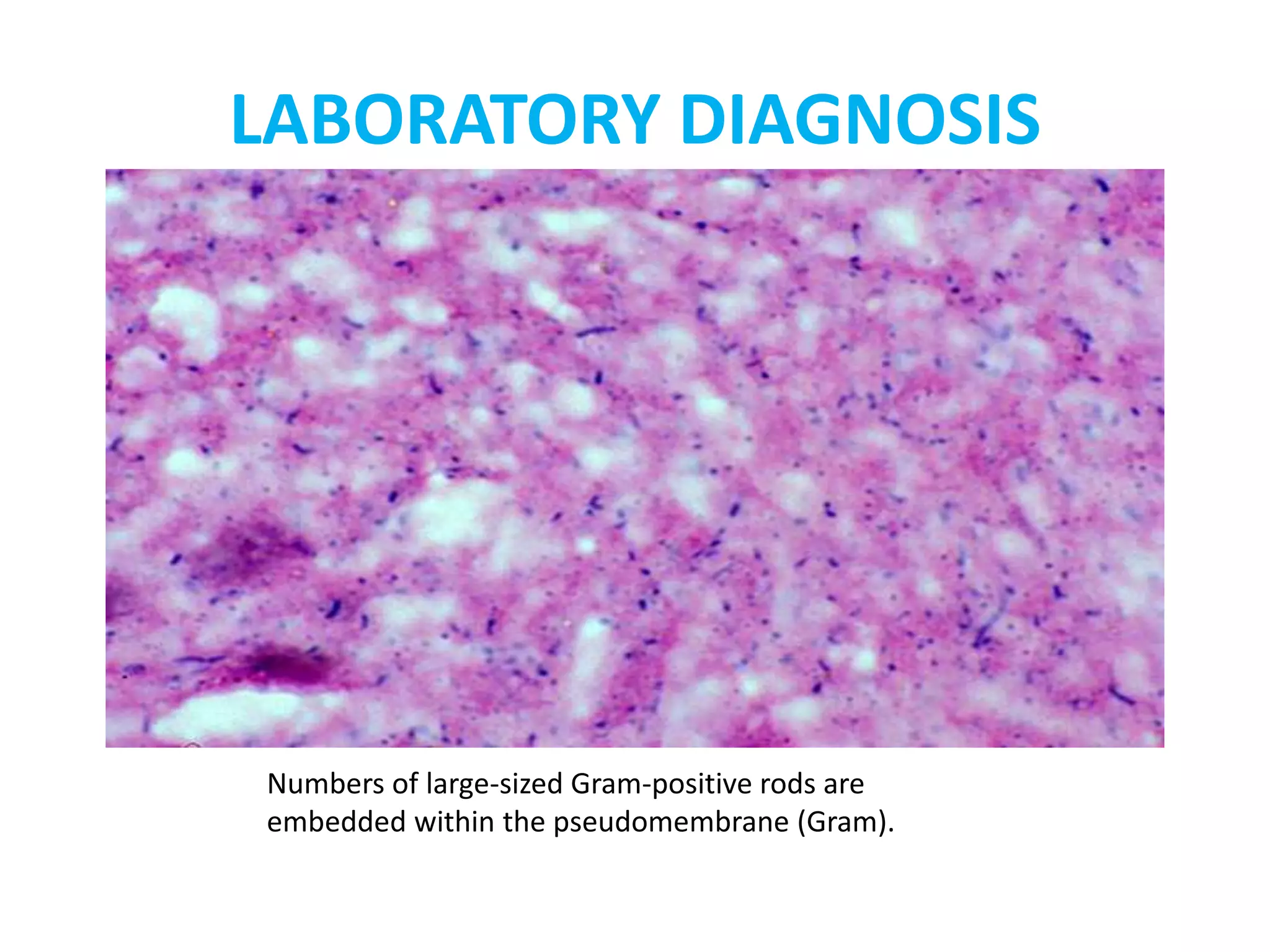
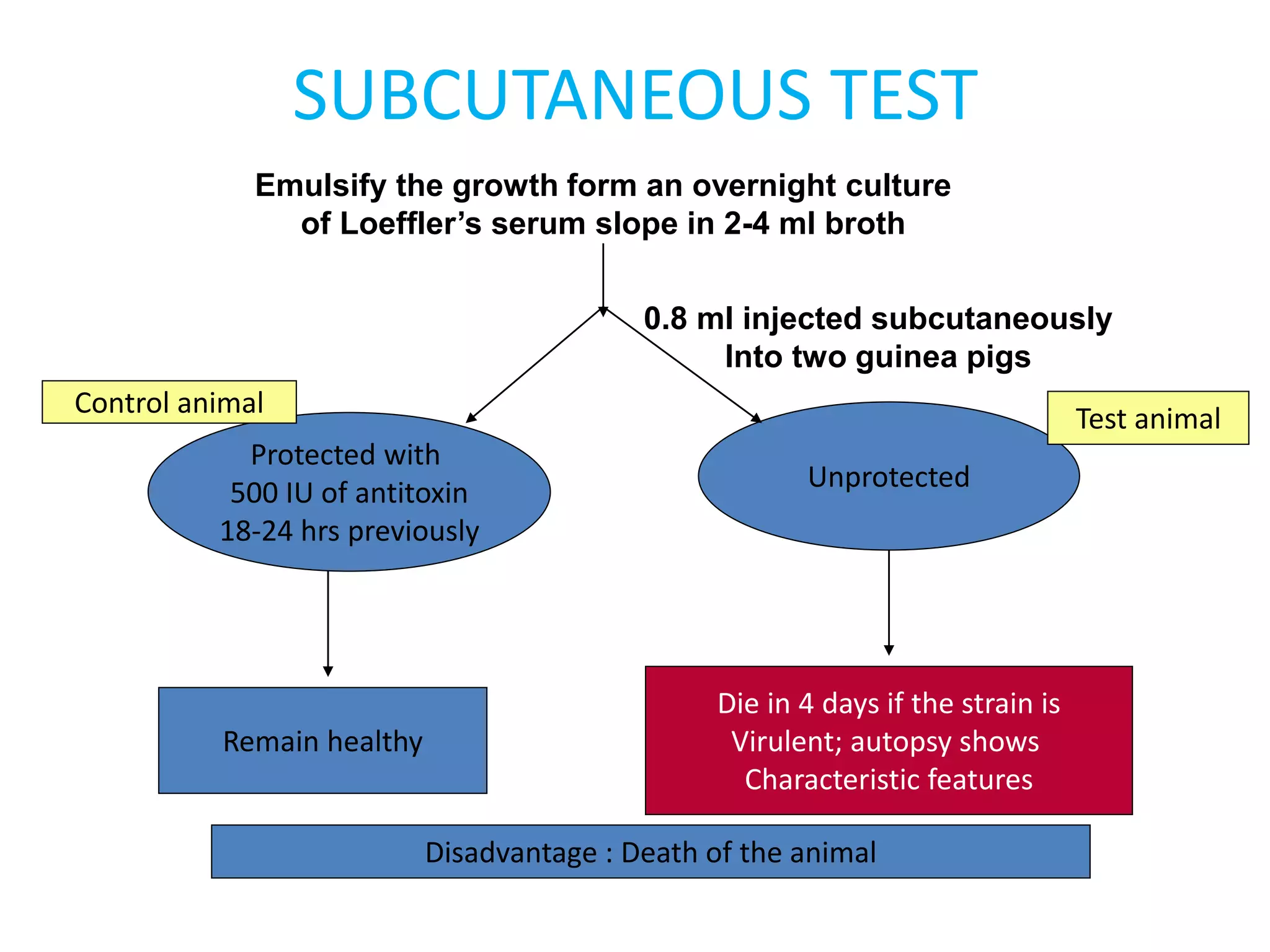
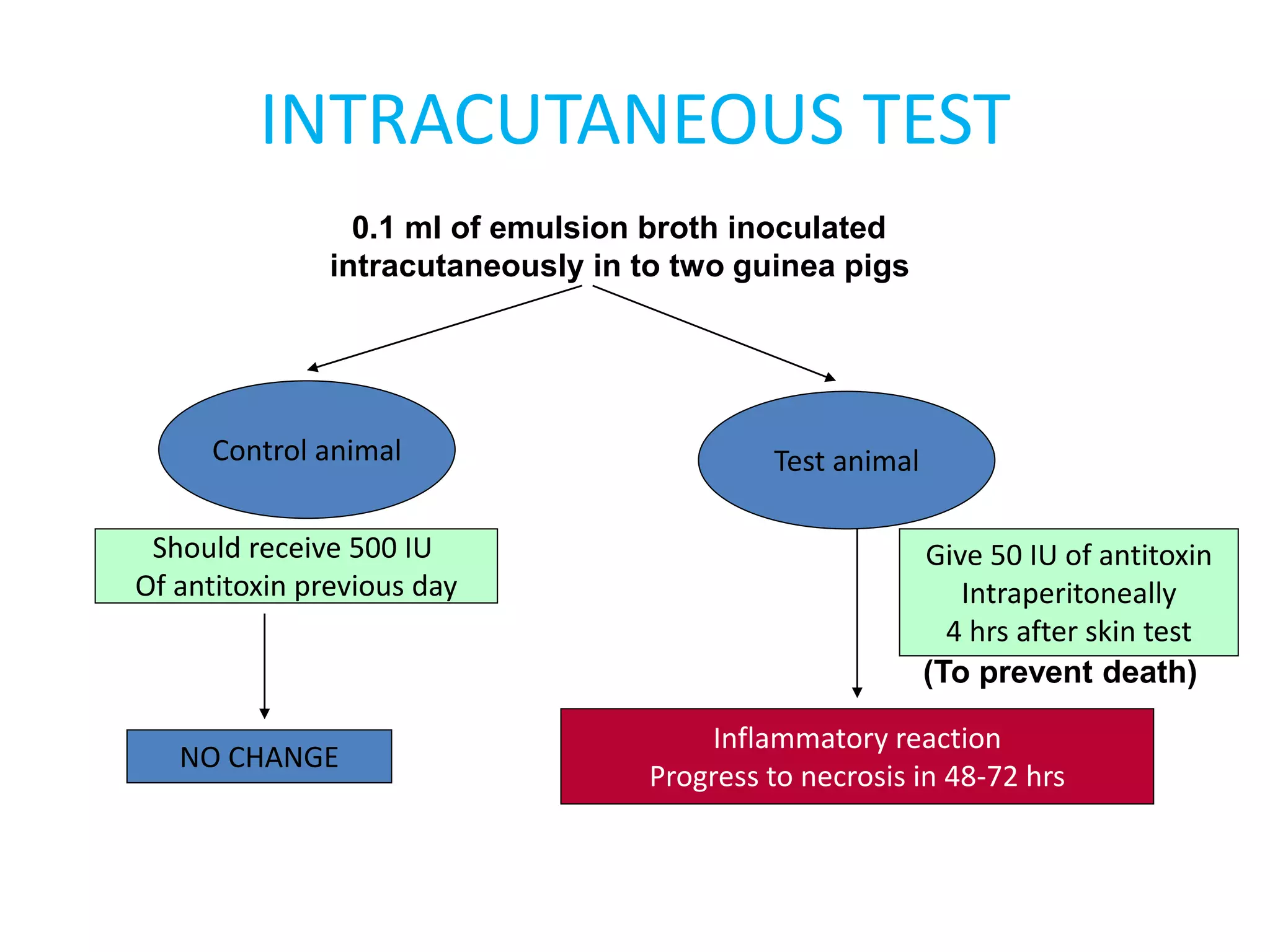
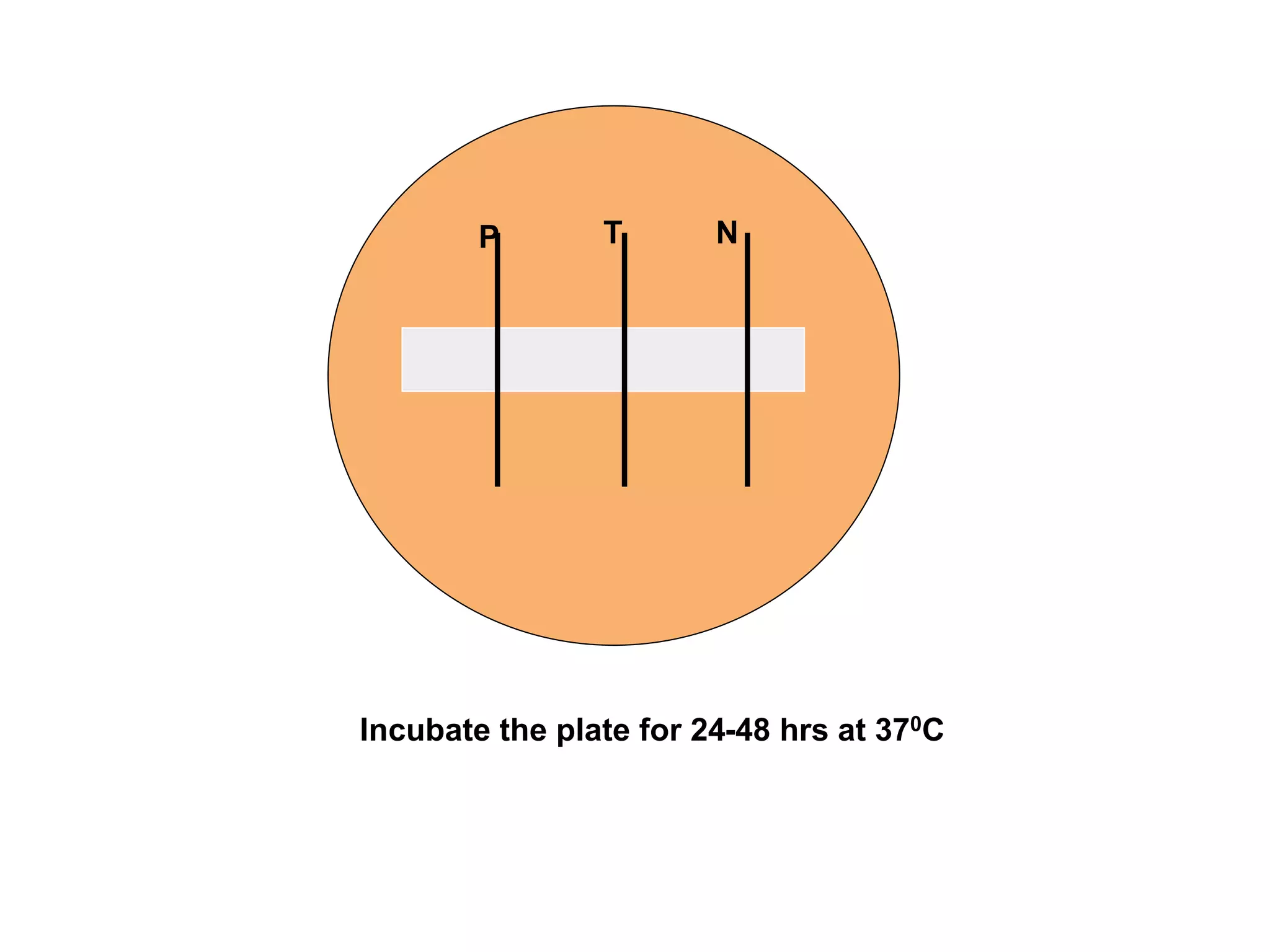
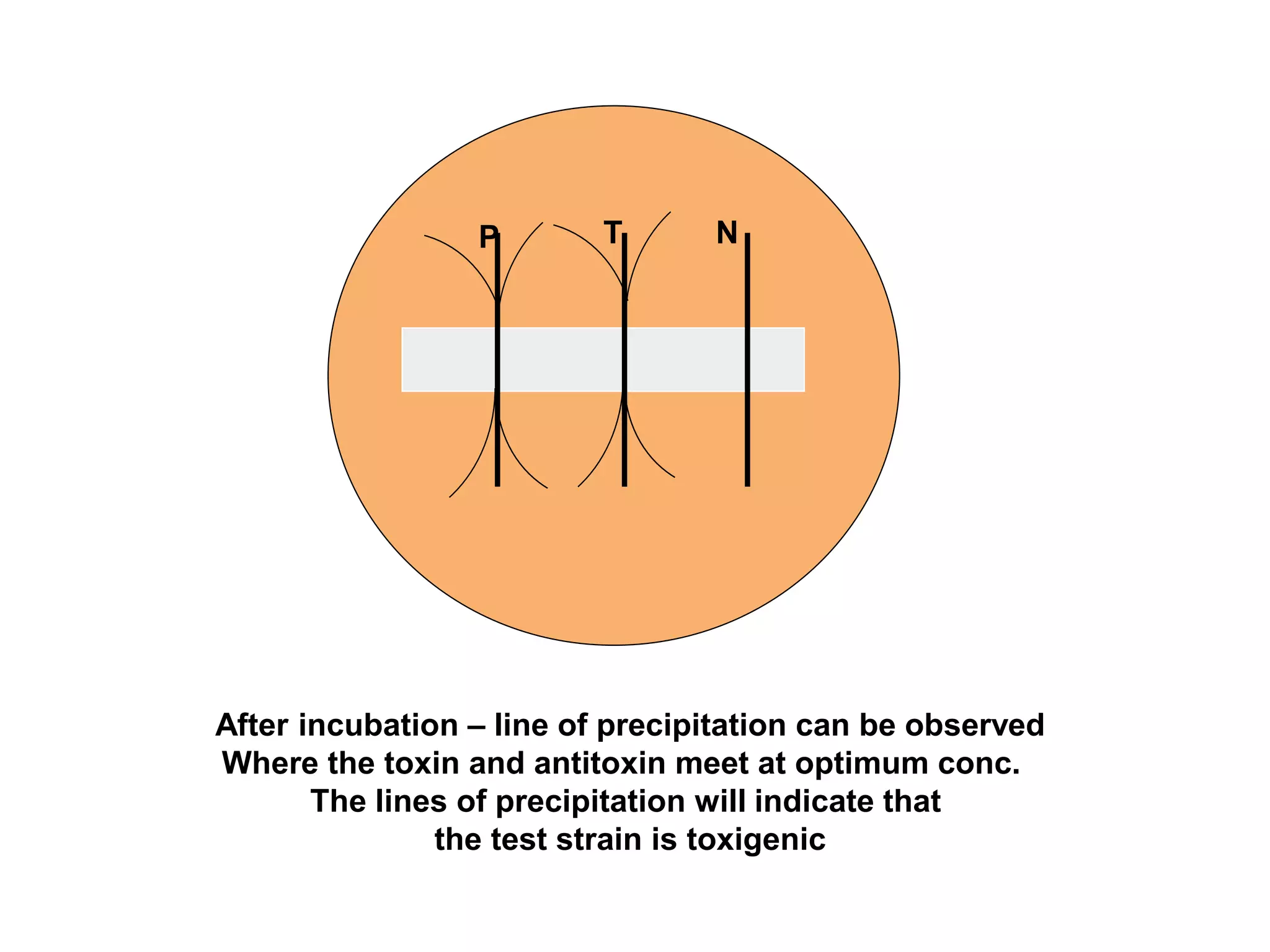
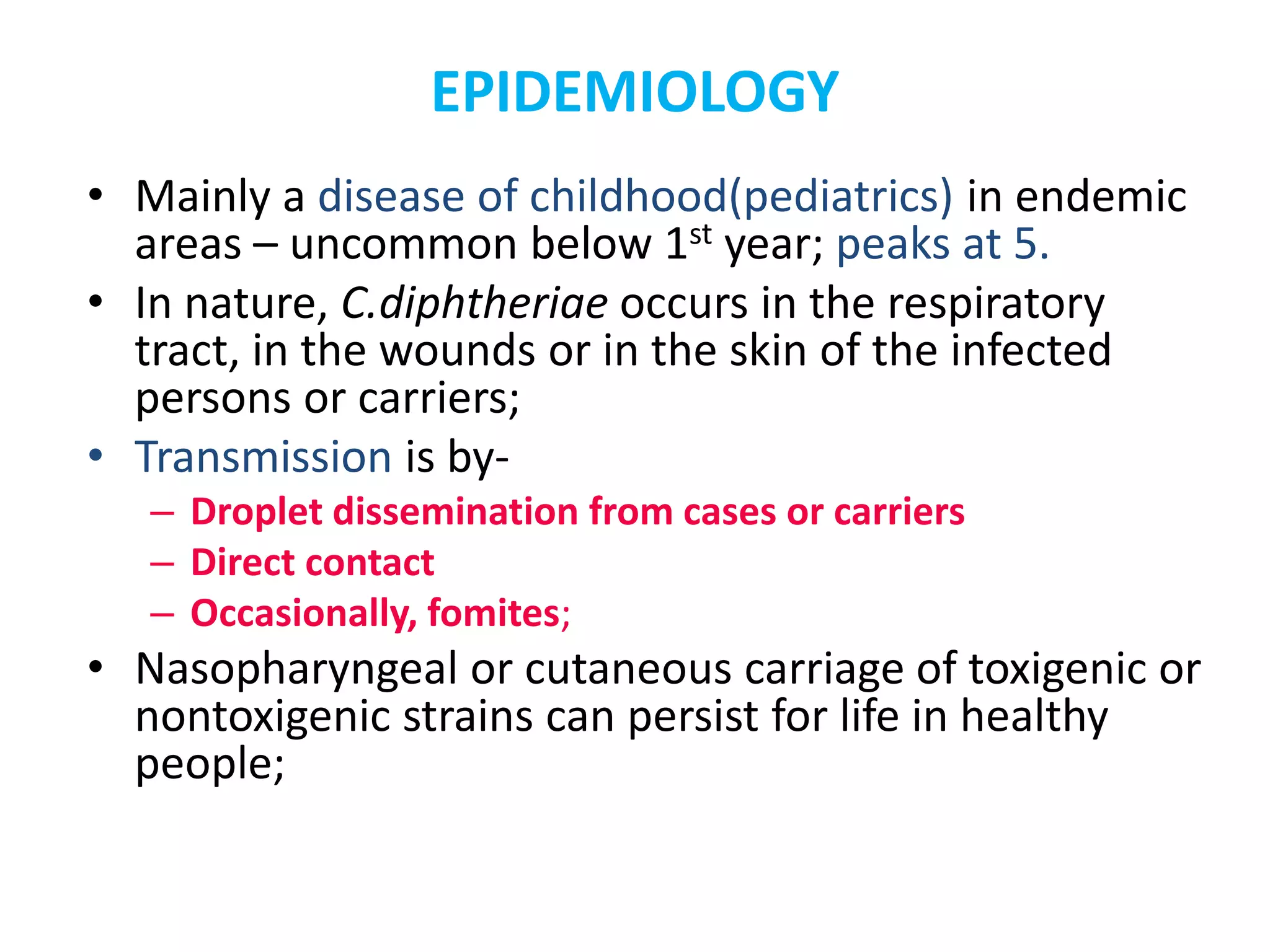
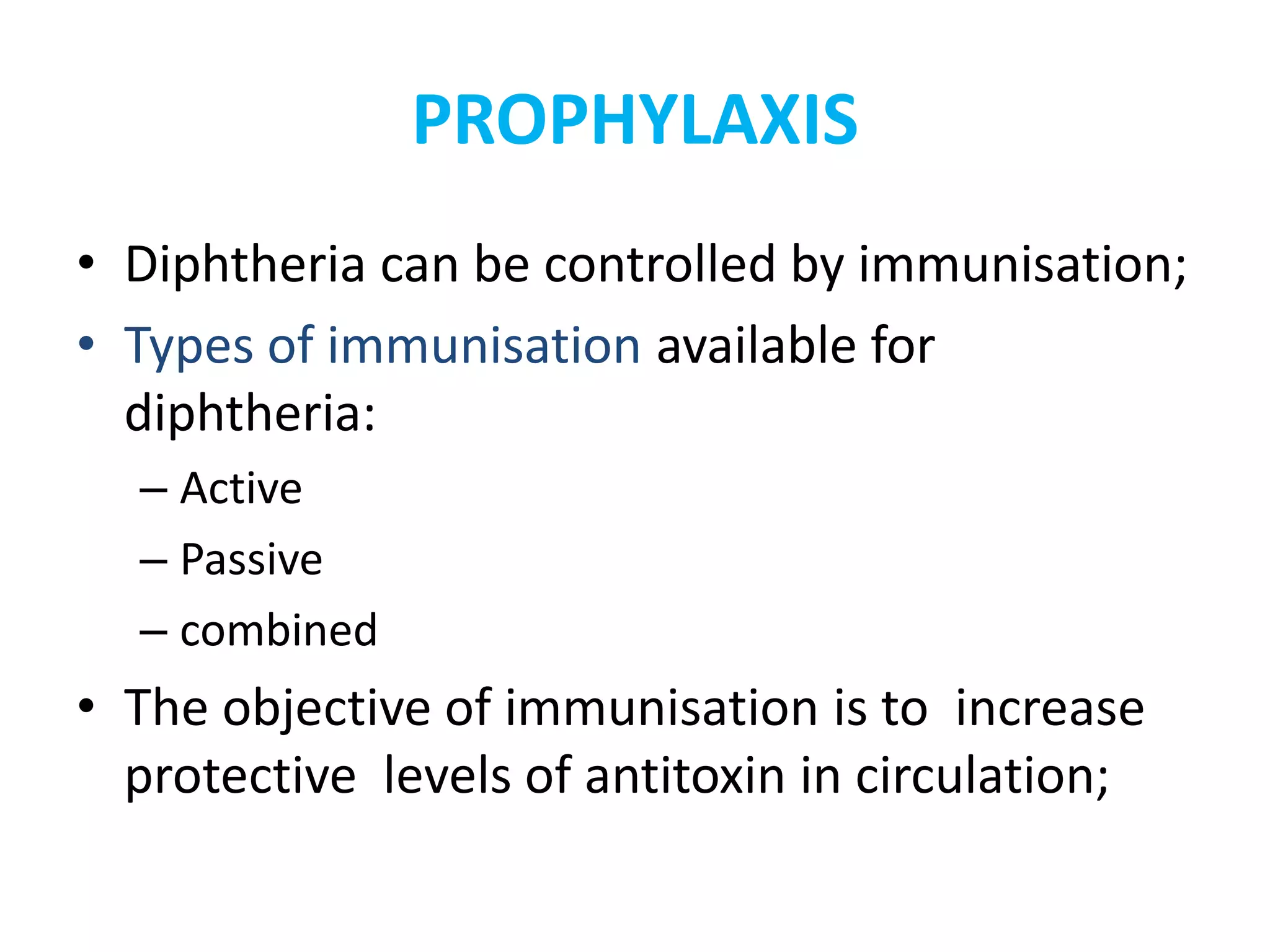
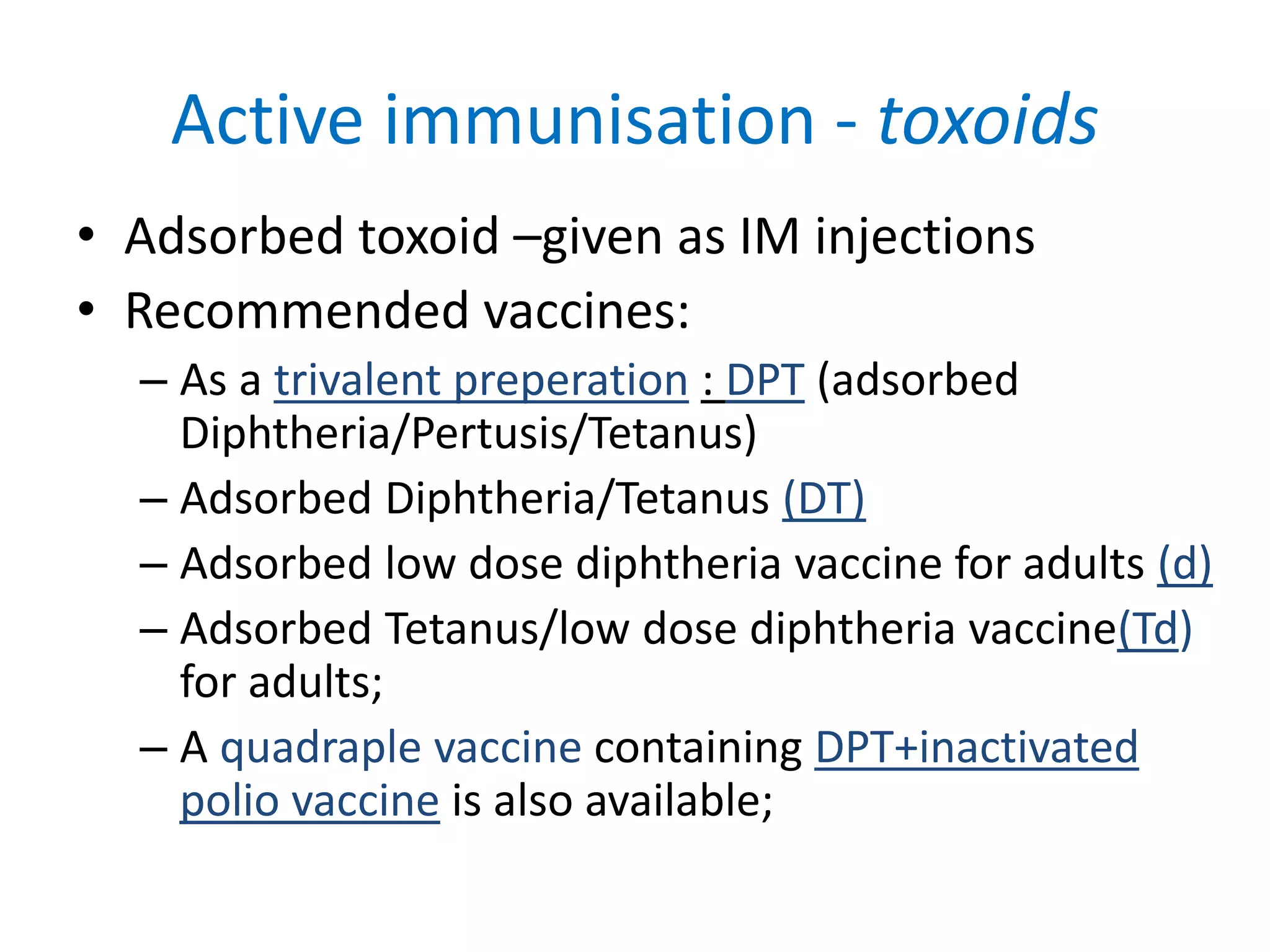
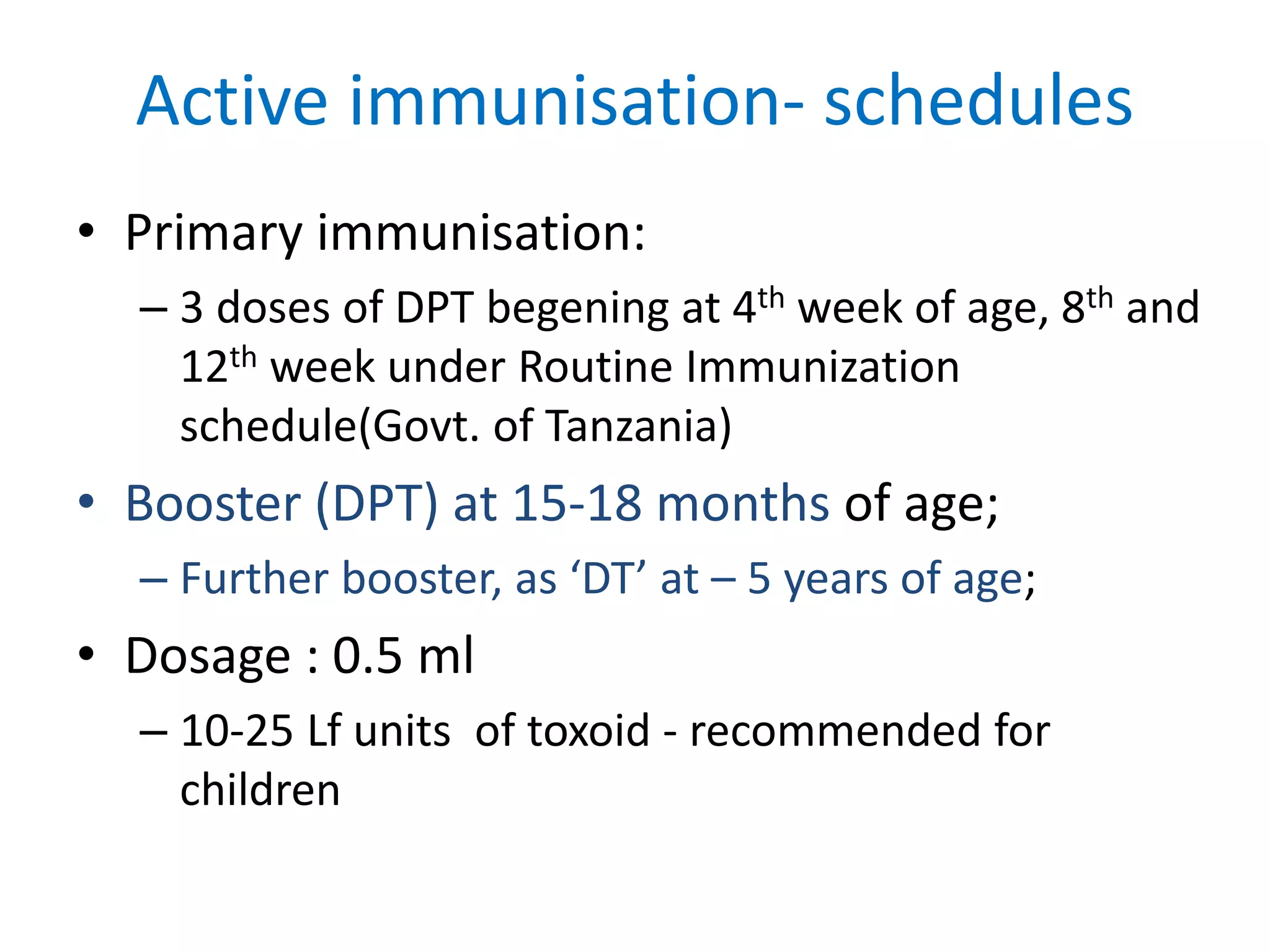
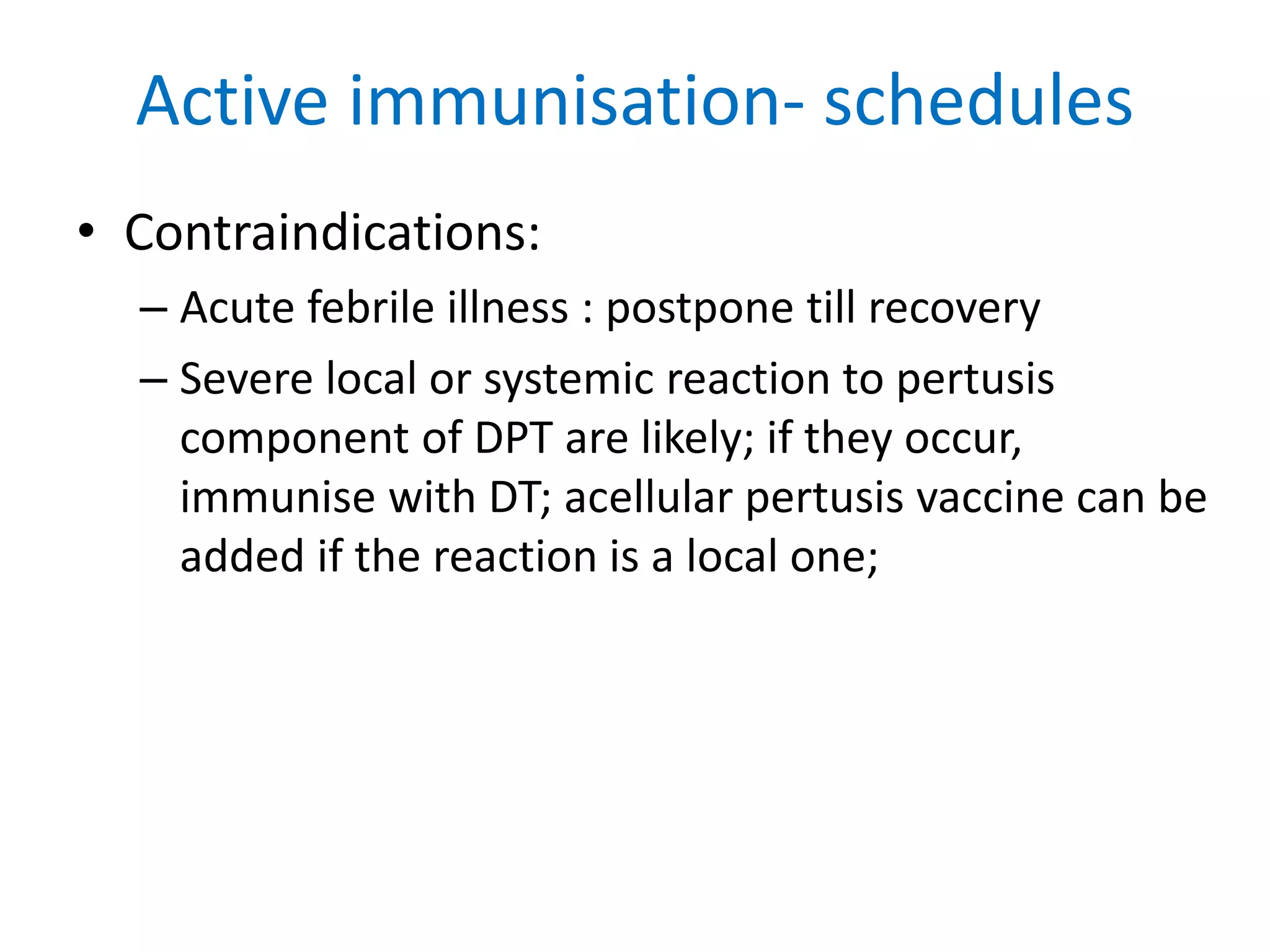
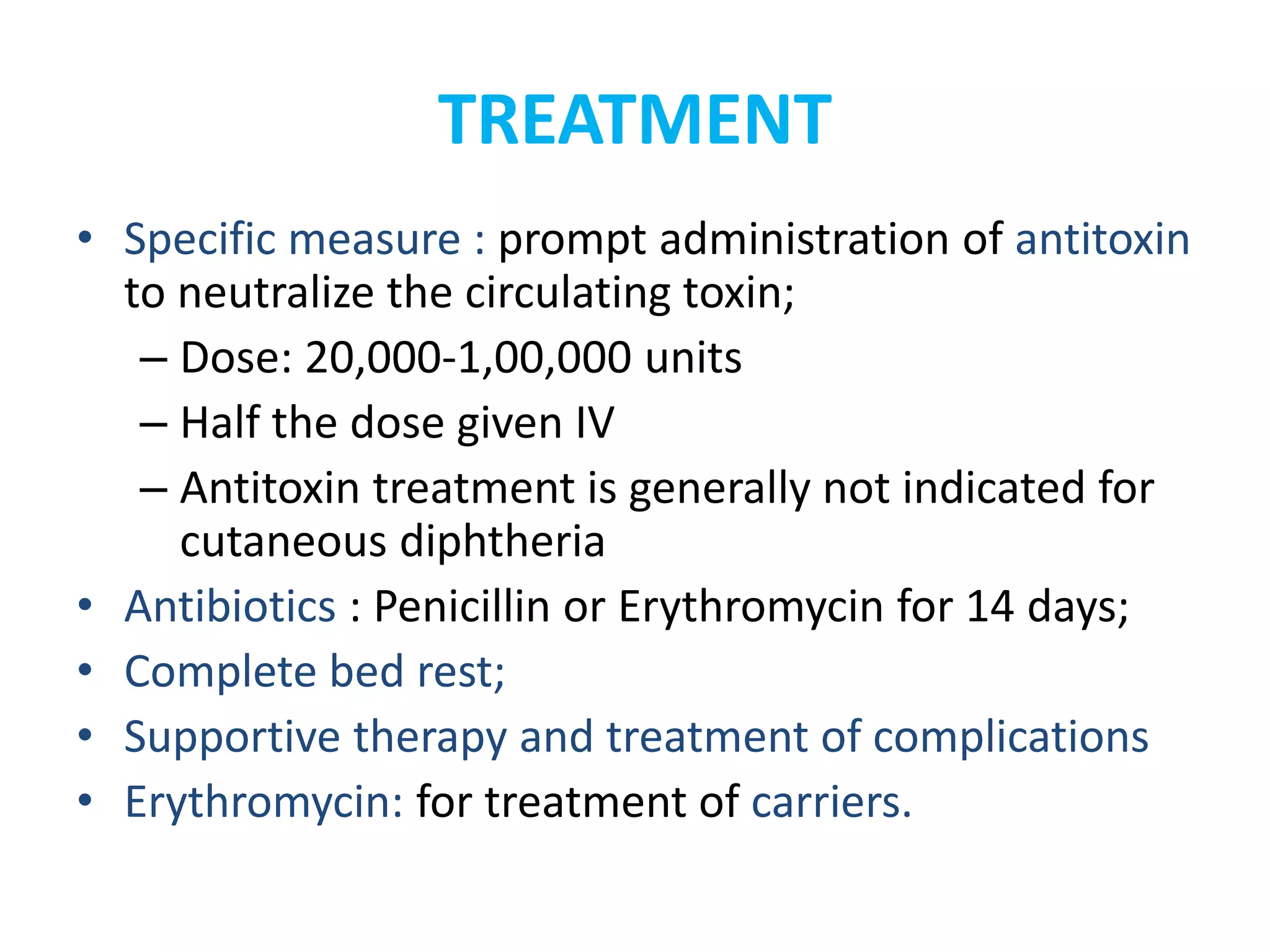
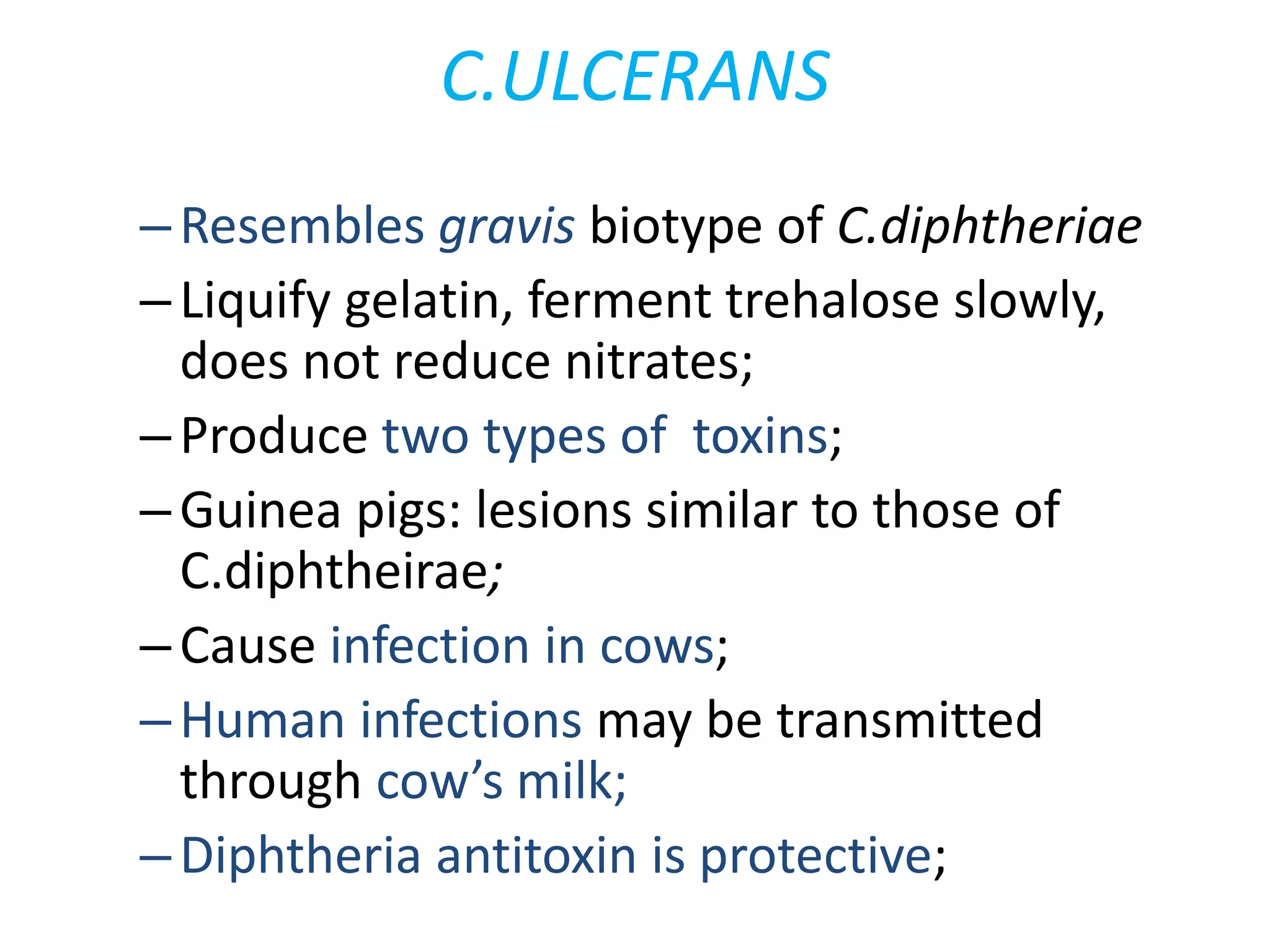
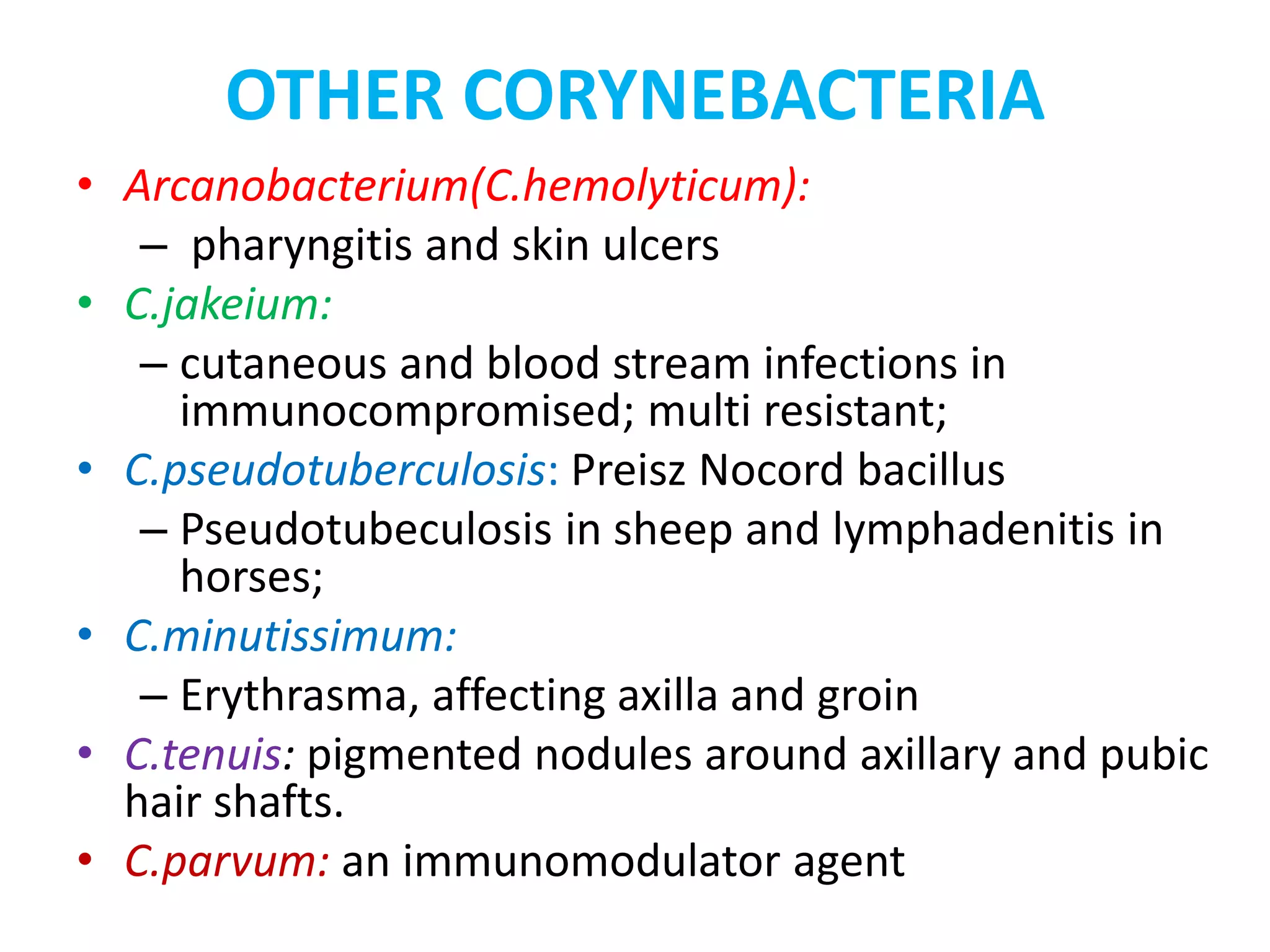
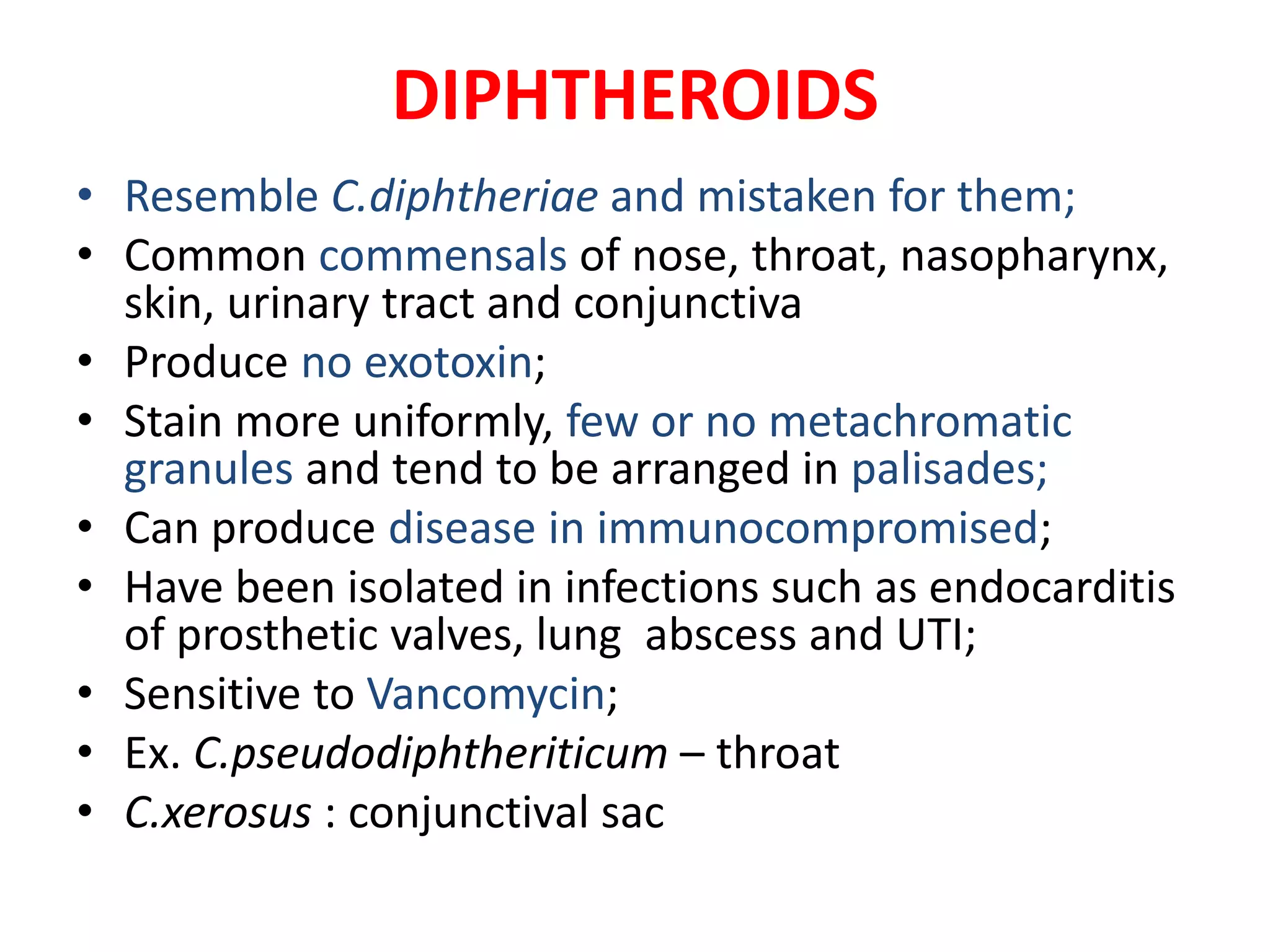
This document provides information on Corynebacterium, including Corynebacterium diphtheriae which causes diphtheria. It discusses the morphology, cultural characteristics, biotypes, virulence factors, pathogenesis, clinical presentation, complications, laboratory diagnosis and epidemiology of C. diphtheriae. The key points are that C. diphtheriae is a gram-positive bacillus that produces a powerful exotoxin causing diphtheria, a serious infection of the upper respiratory tract, and immunization is important for control of the disease.