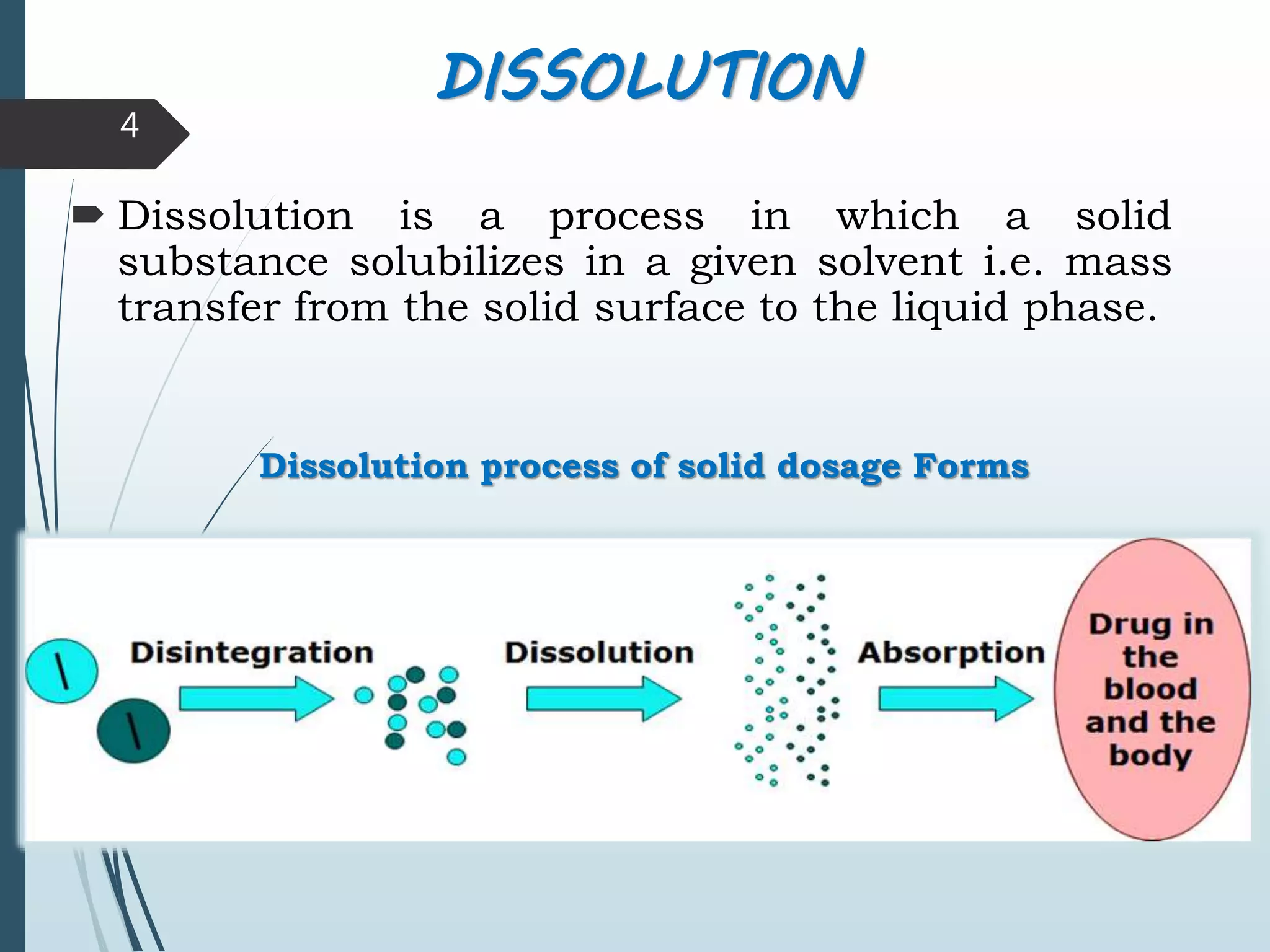
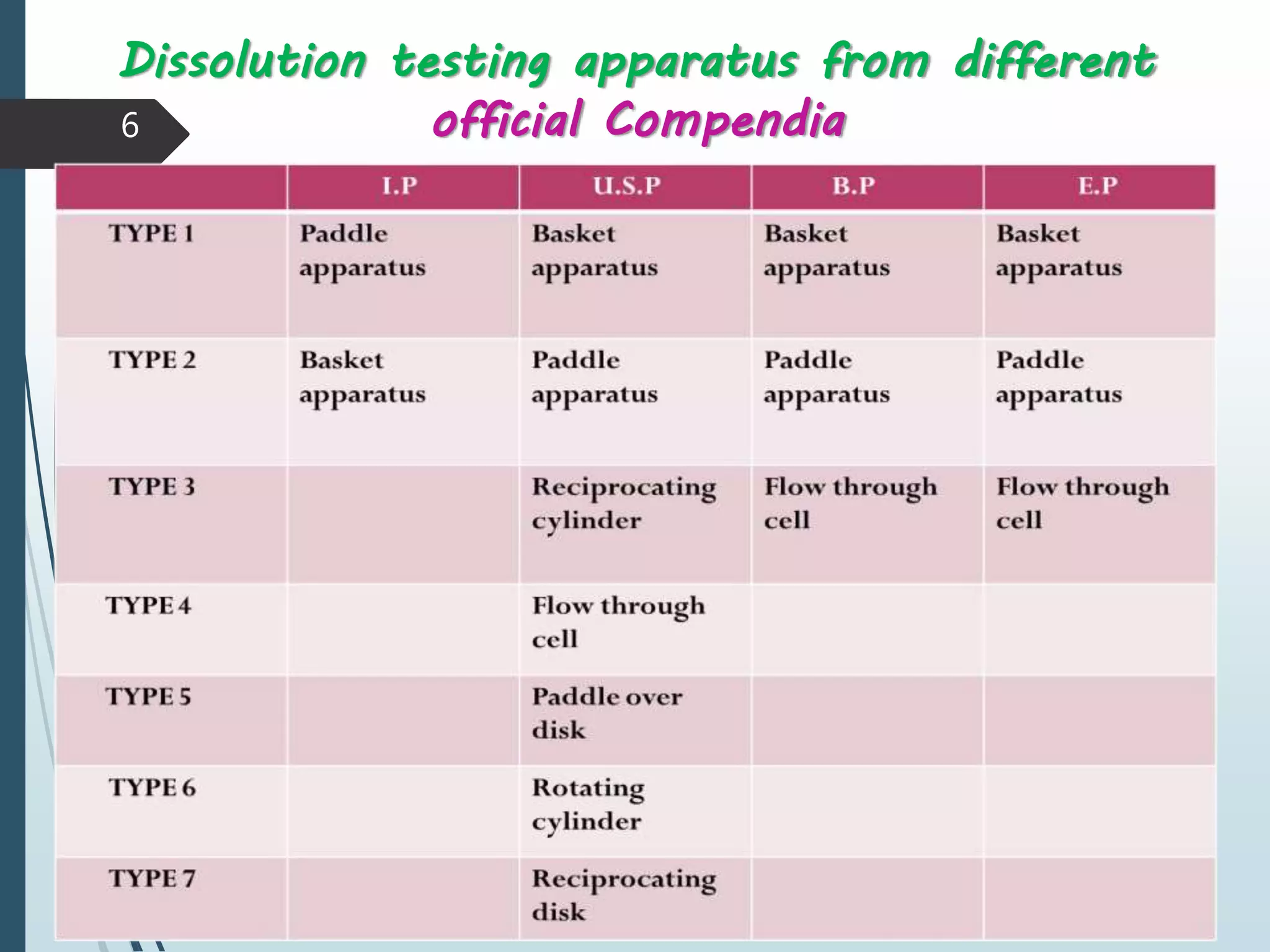
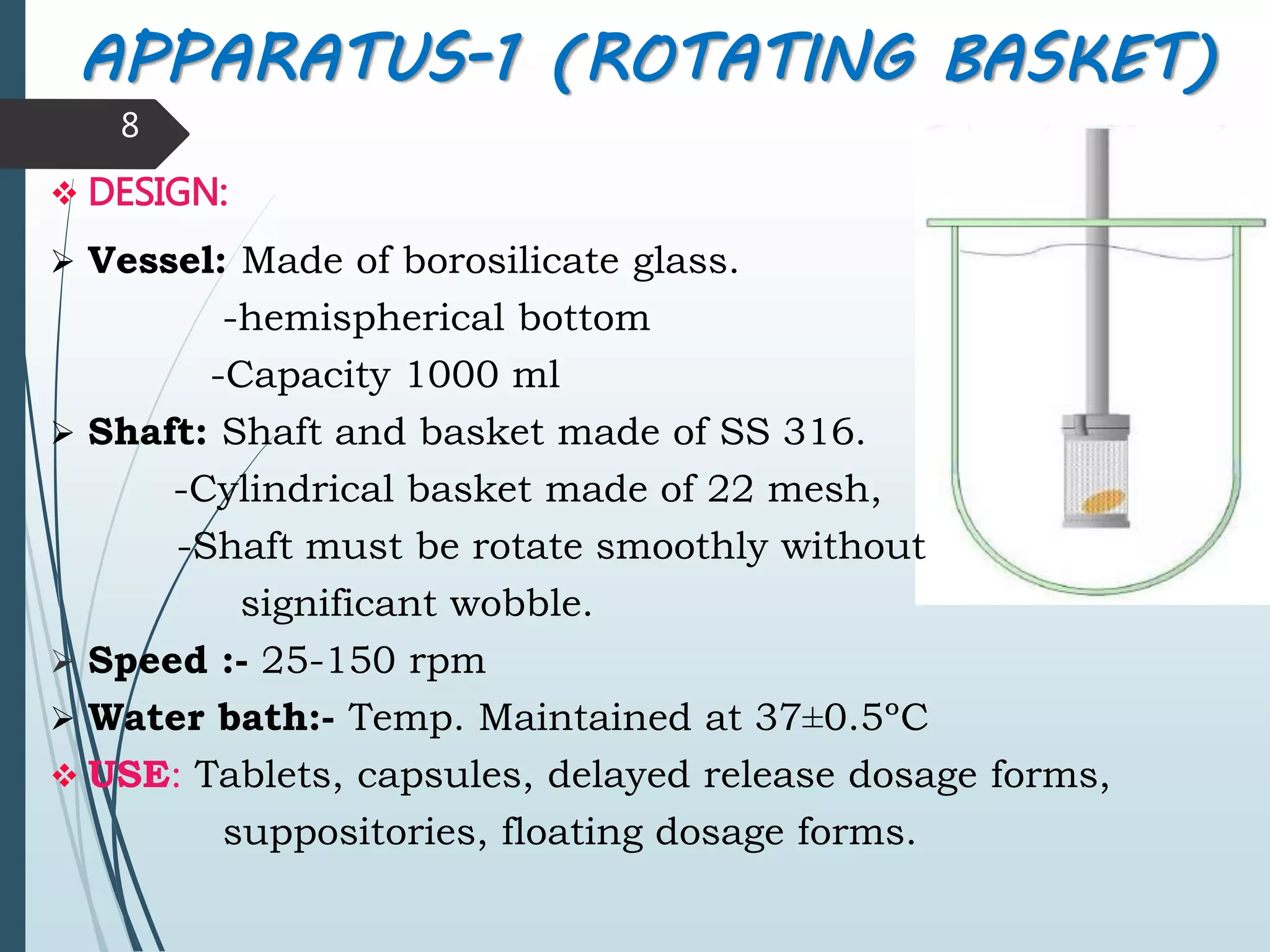
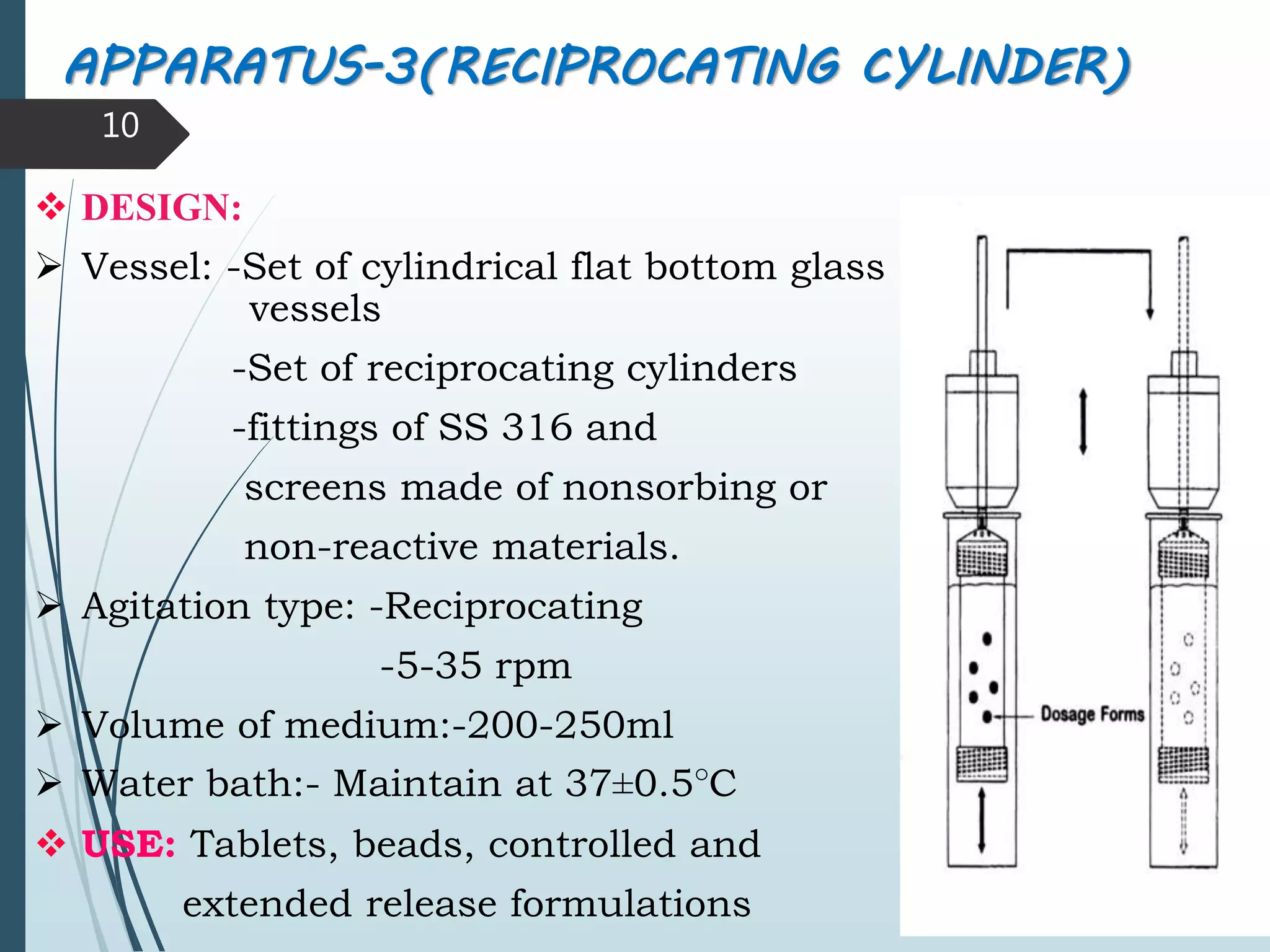
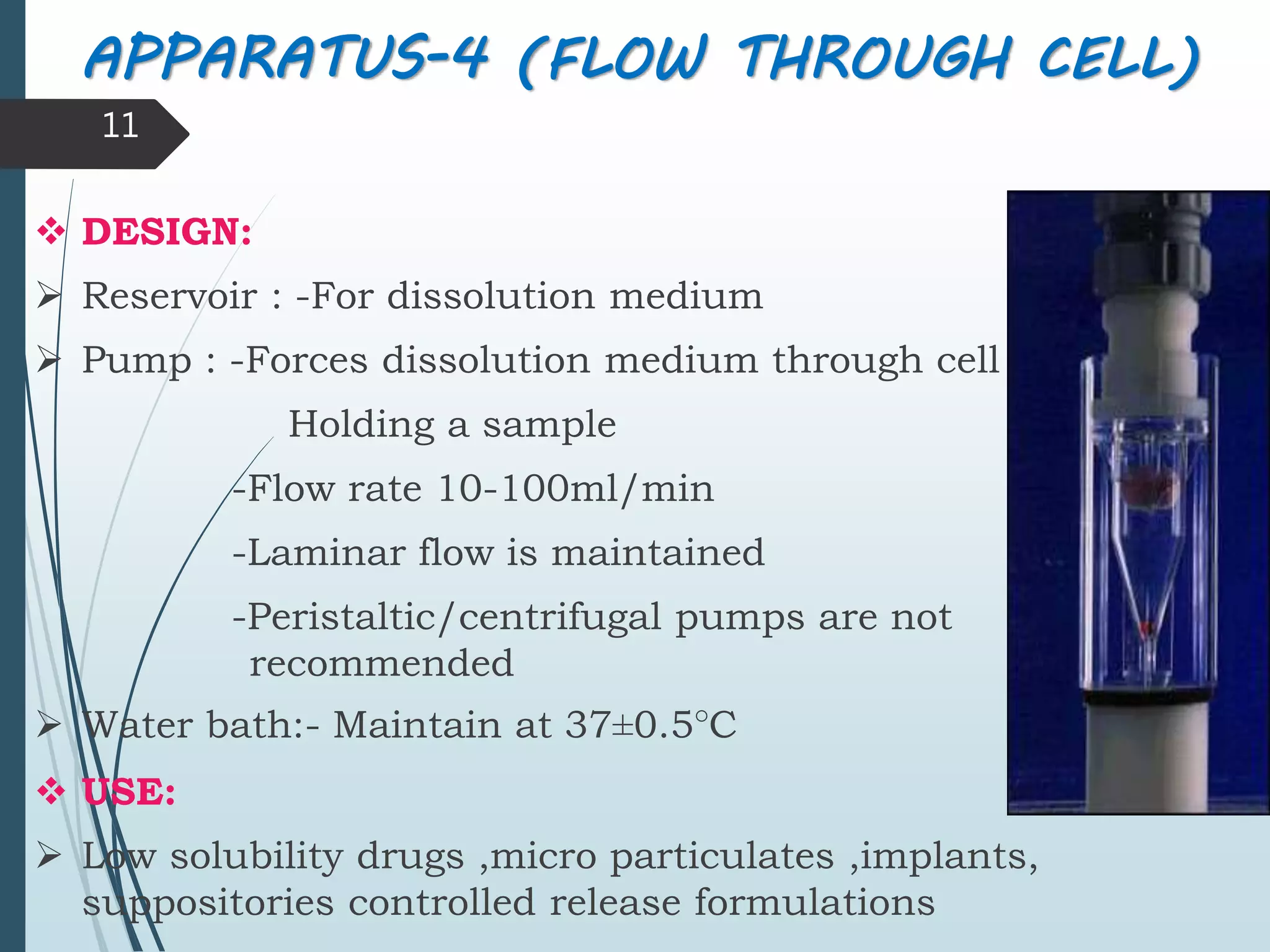
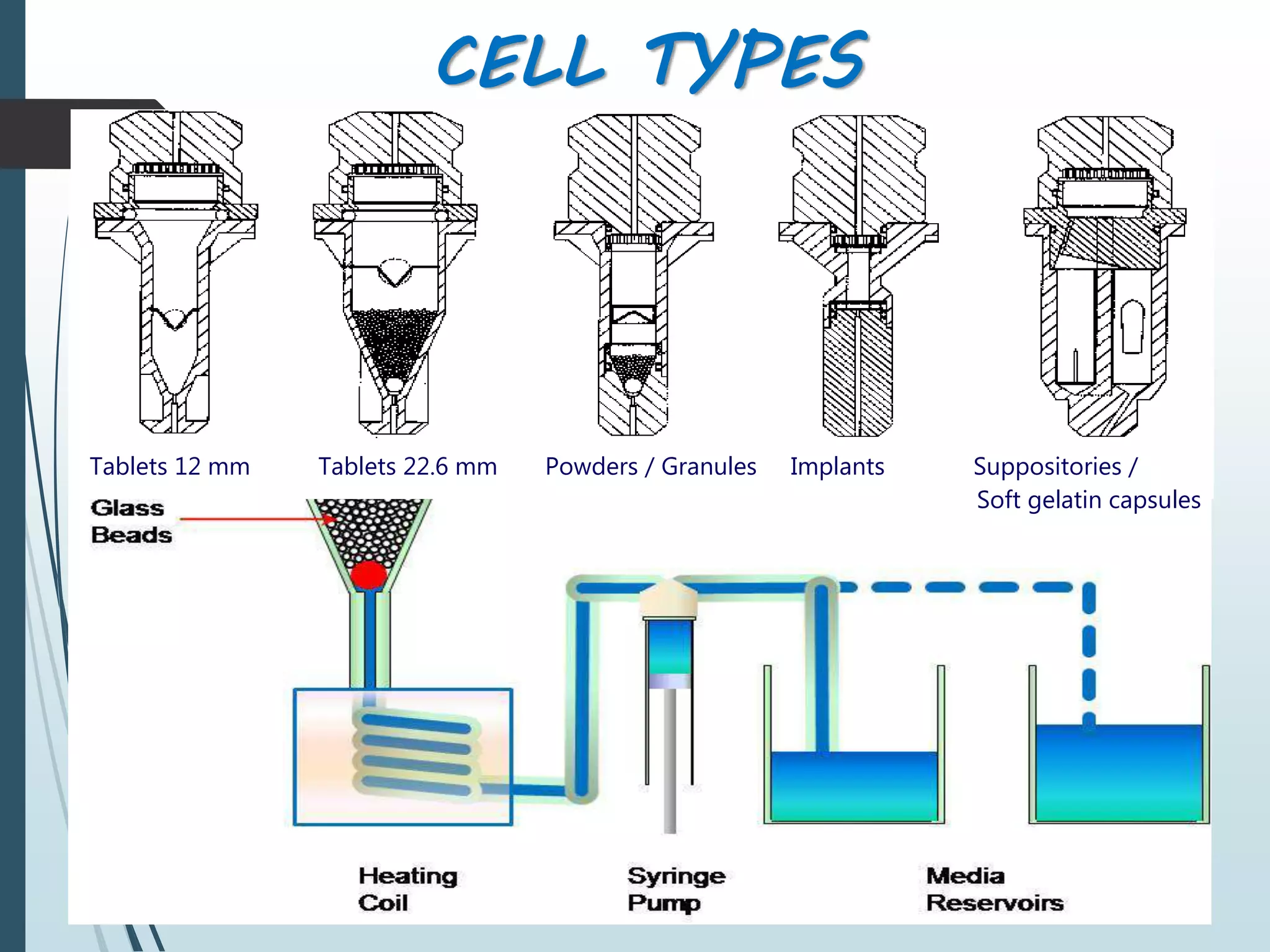
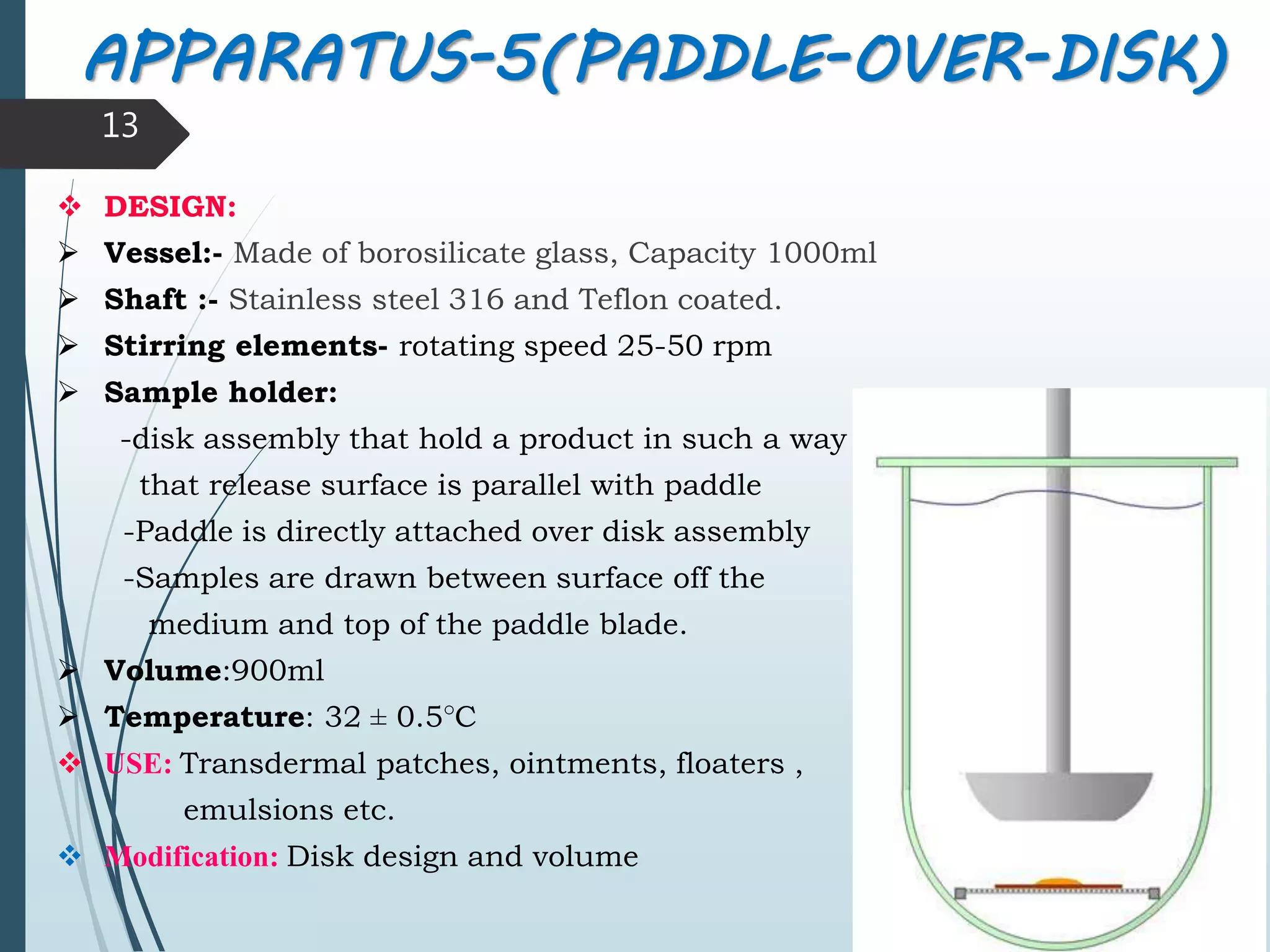
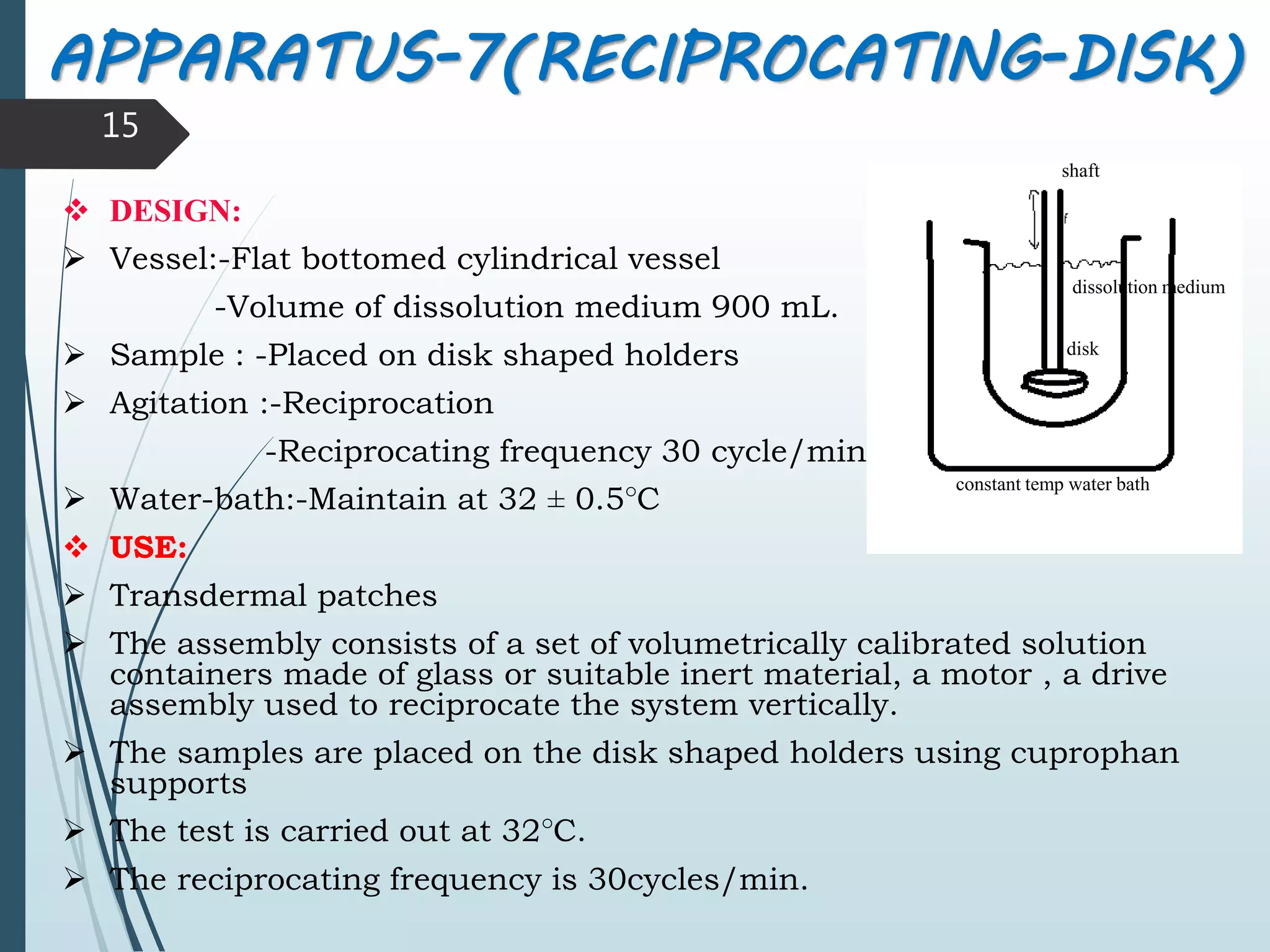
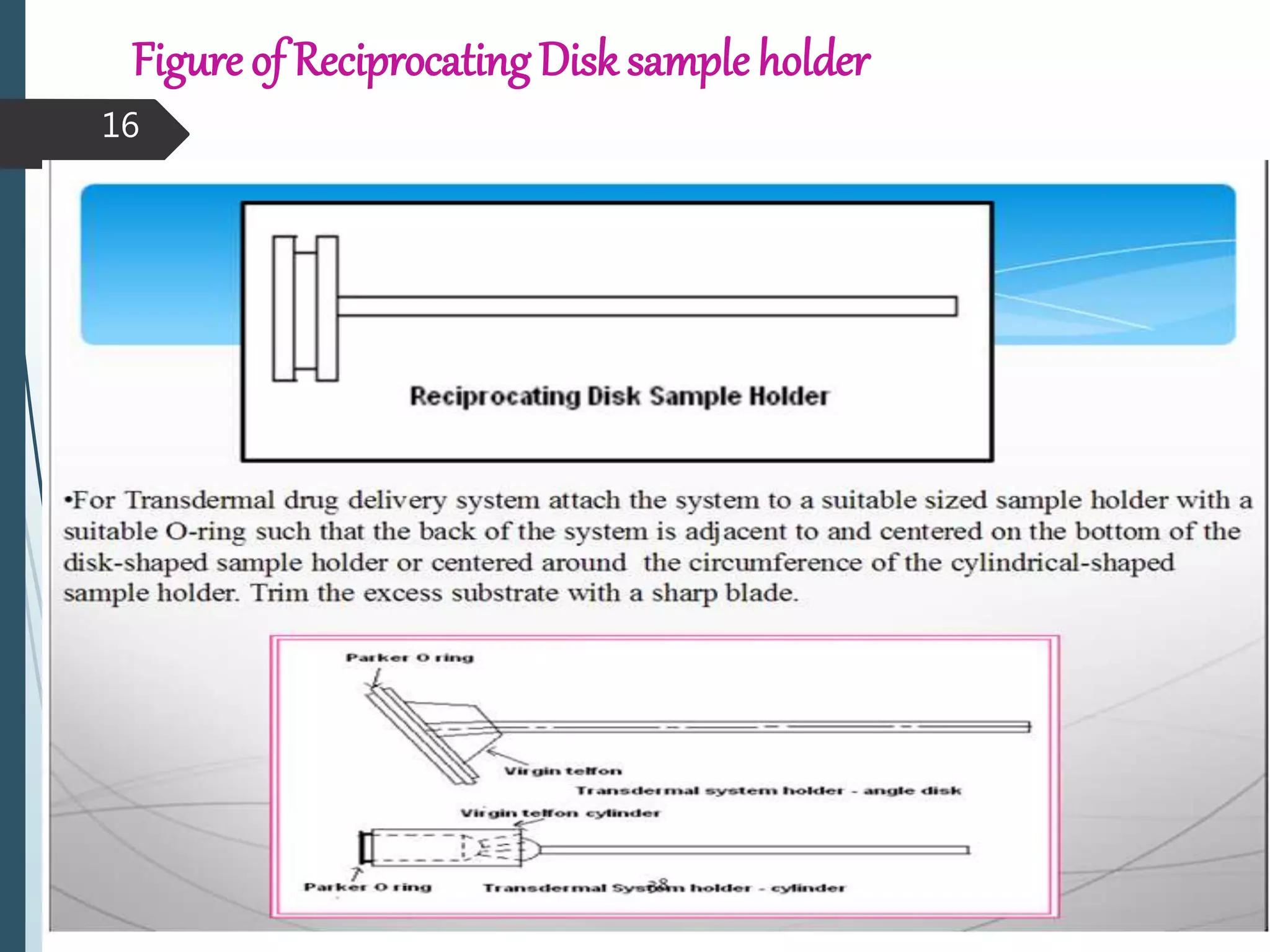
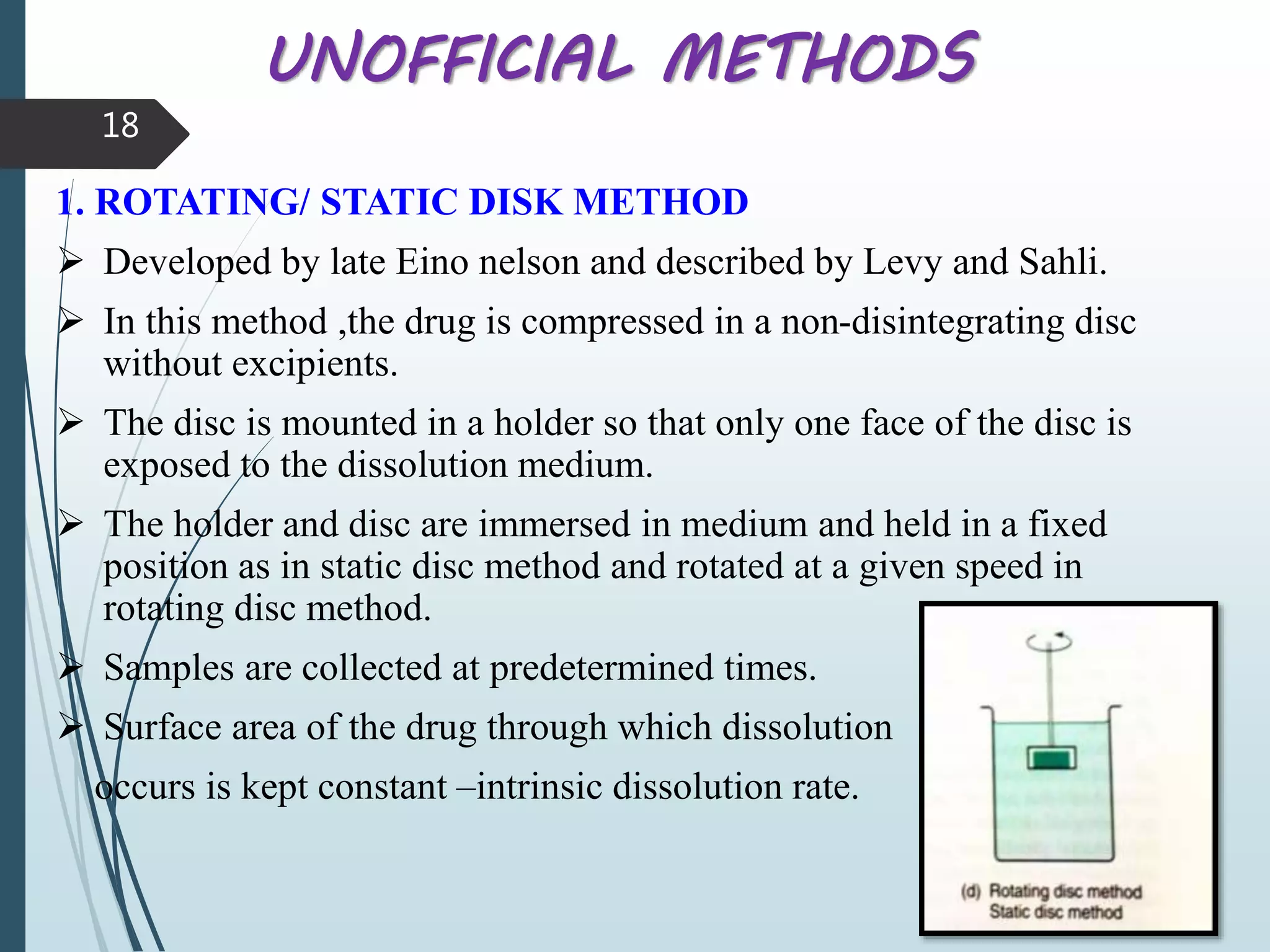
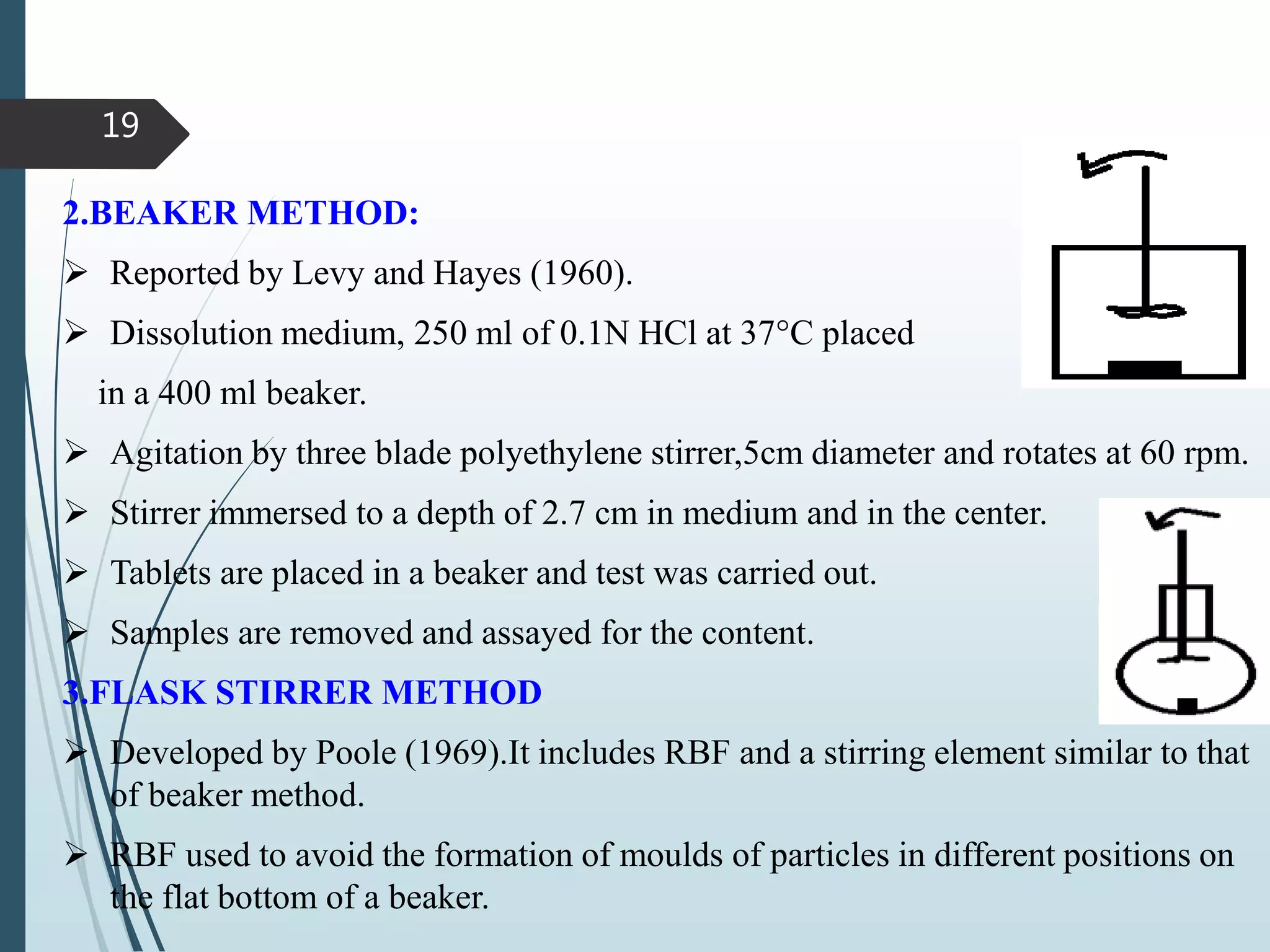
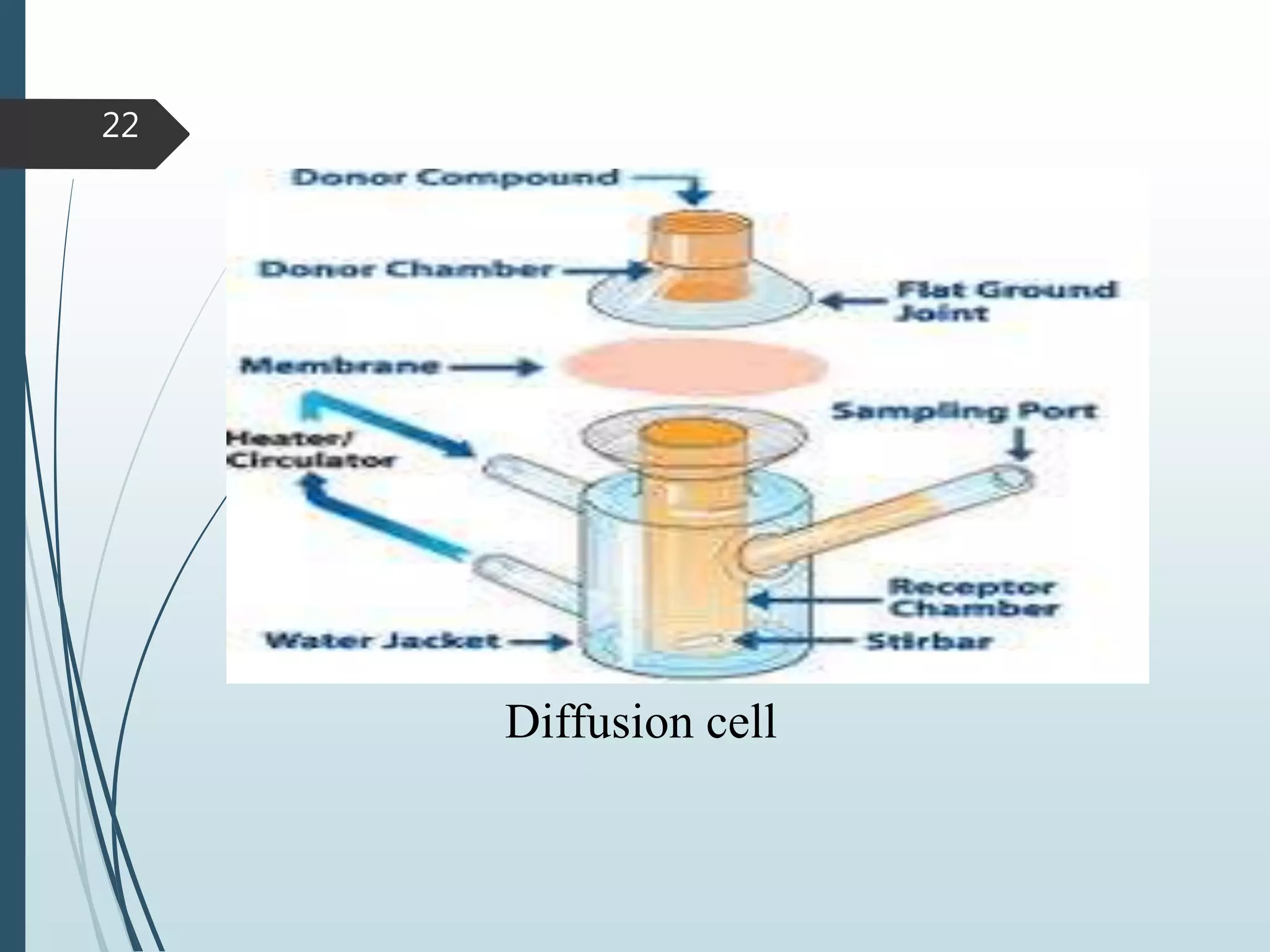
This document discusses dissolution testing, which is an important quality control procedure for pharmaceutical dosage forms. It begins by introducing dissolution testing and explaining that it measures the rate and extent of dissolution of a drug product under specified conditions. It then describes the various apparatus used for dissolution testing according to official compendia like the USP, including the basket, paddle, reciprocating cylinder, flow-through cell, paddle-over-disk, rotating cylinder, and reciprocating disk methods. The document also discusses factors that can influence dissolution and concludes that dissolution testing is a valuable tool for evaluating batch-to-batch consistency and biological availability of drugs from formulations.