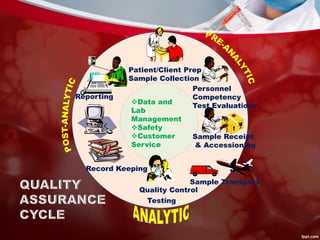
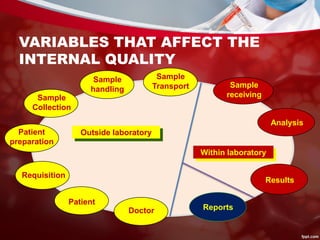
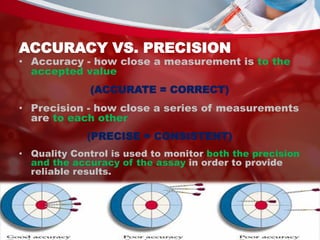
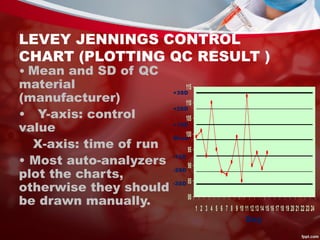
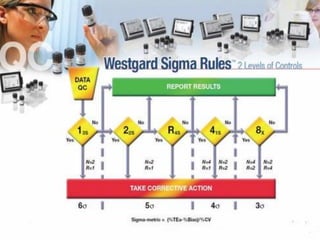
This document discusses quality control in laboratories. It defines key terms like quality assurance, quality assessment, total quality management, and continuous quality improvement. It describes factors that can affect quality like pre-analytical, analytical, and post-analytical variables. The importance of standard operating procedures, proficiency testing, and documenting quality control procedures is emphasized. Maintaining accurate and precise results through internal quality control using control charts and Westgard rules is also outlined.