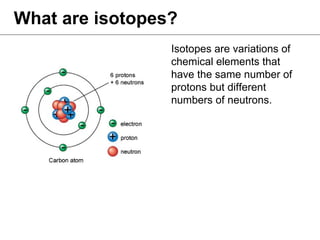
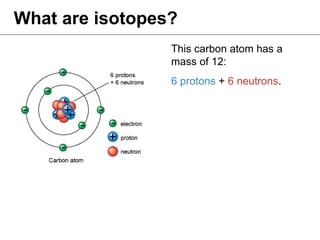
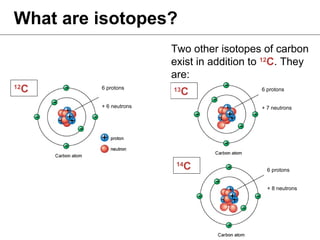
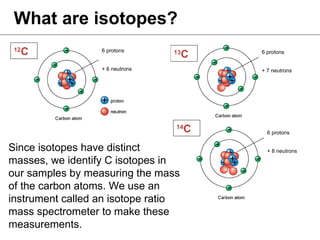
Isotopes are variations of chemical elements that have the same number of protons but different numbers of neutrons. We identify isotopes based on their mass, which is the sum of protons and neutrons. Carbon has three main isotopes: 12C with 6 protons and 6 neutrons; 13C with 6 protons and 7 neutrons; and 14C with 6 protons and 8 neutrons. Isotopes are identified by their distinct masses, which are measured using an isotope ratio mass spectrometer.