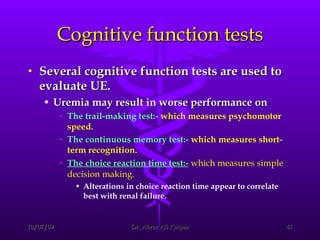
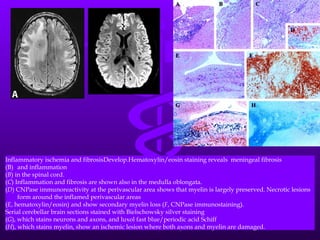
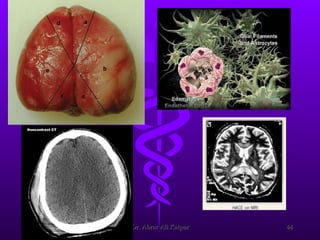
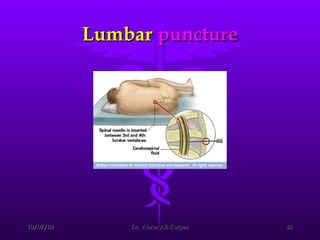
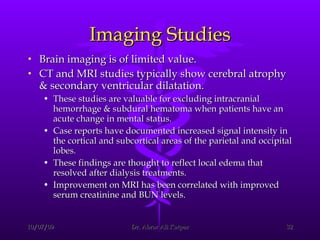
Uremic encephalopathy occurs when toxins that are normally cleared by the kidneys build up in the bloodstream due to kidney failure. It causes a range of neurological symptoms from mild issues like fatigue to severe problems like seizures and coma. The condition develops when kidney function declines to the point that creatinine clearance levels fall below 15 mL/min. While the exact cause is unknown, it involves the accumulation of various toxins in the brain that disrupts cell metabolism and function. Prompt treatment with dialysis or kidney transplantation can reverse the neurological symptoms.



























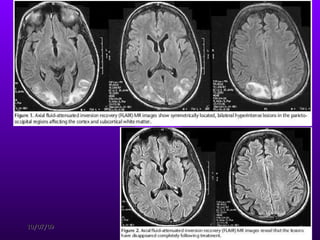

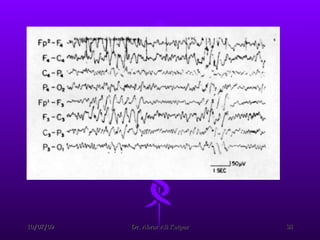
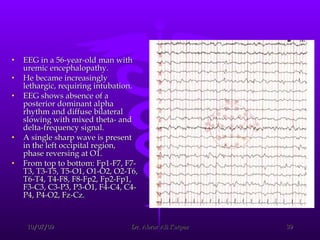
![Reduction in frequency of EEG waves correlates with the decrease in renal function and the alterations in cerebral function. After the initial period of dialysis, clinical stabilization may occur while the EEG findings do not improve. Eventually, EEG results move toward normal. Aside from the routine EEG, evoked potentials (EPs) (ie, EEG signals that occur at a reproducible time after the brain receives a sensory stimulus [eg, visual, auditory, somatosensory]) may be helpful in evaluating uremic encephalopathy. CRF prolongs latency of the cortical visual-evoked response. Auditory-evoked responses are generally not altered in uremia, but delays in the cortical potential of the somatosensory-evoked response do occur.](https://image.slidesharecdn.com/uremicencephalopathy-091007163208-phpapp02/85/Uremic-Encephalopathy-40-320.jpg)