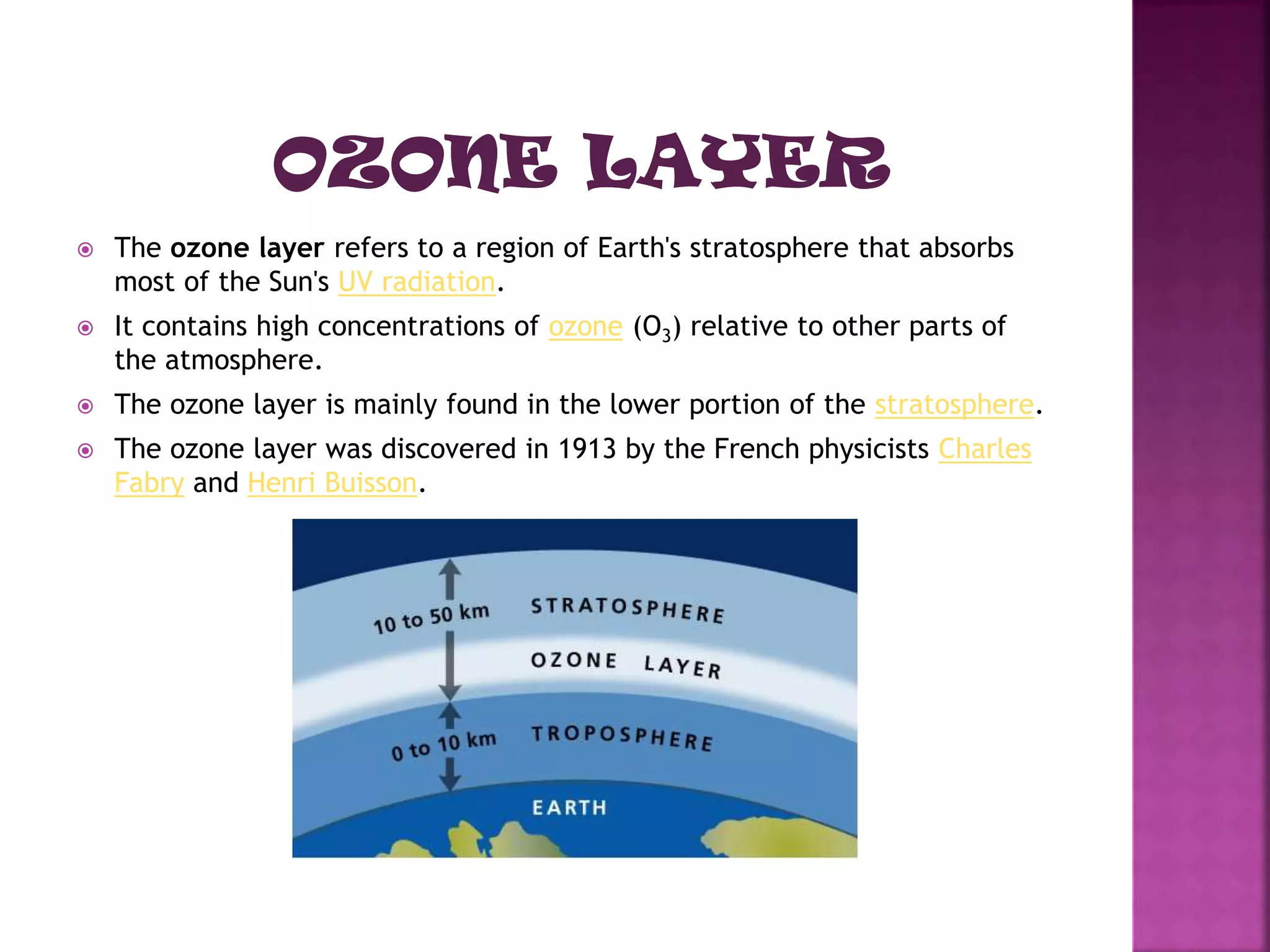
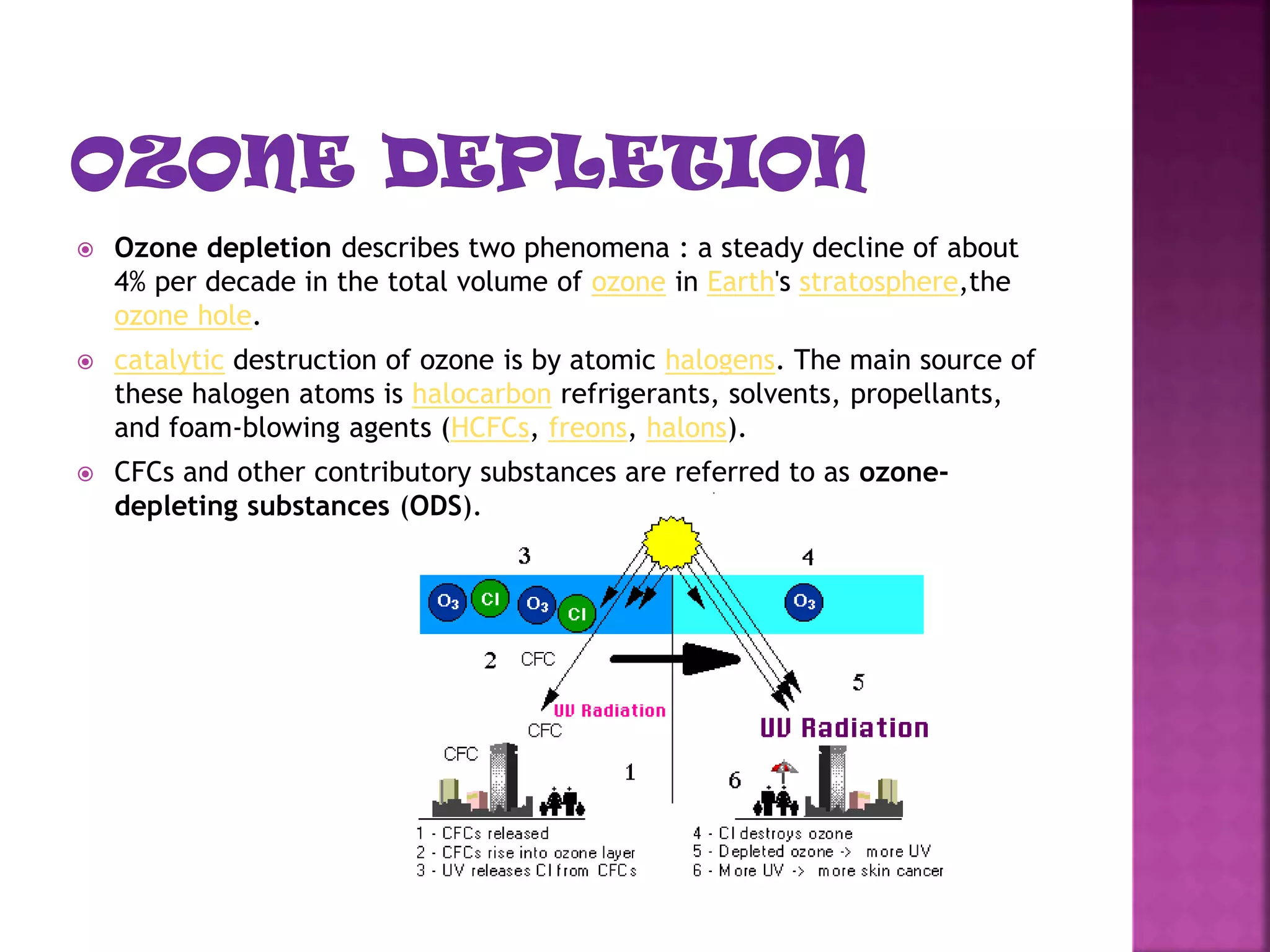
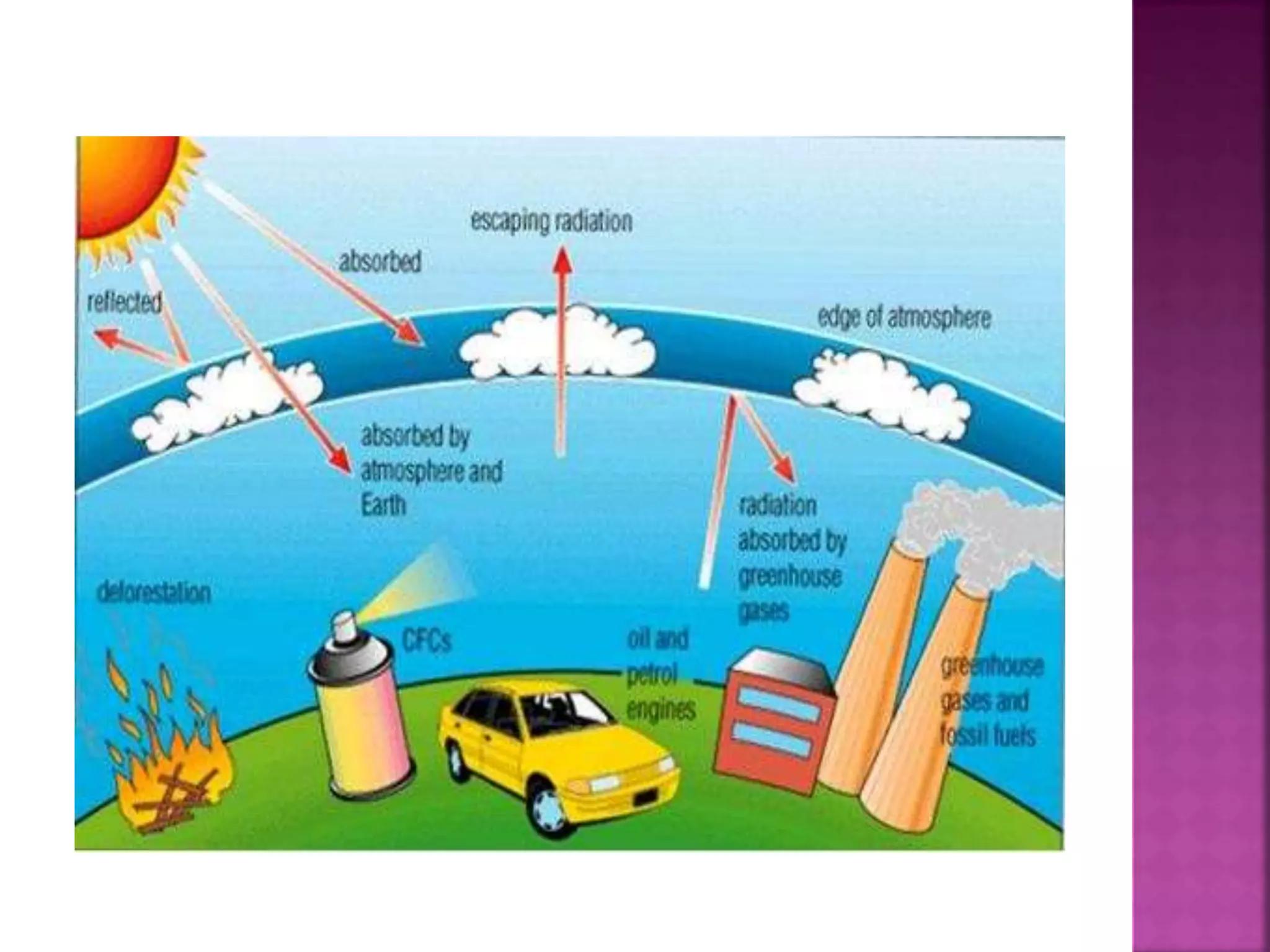
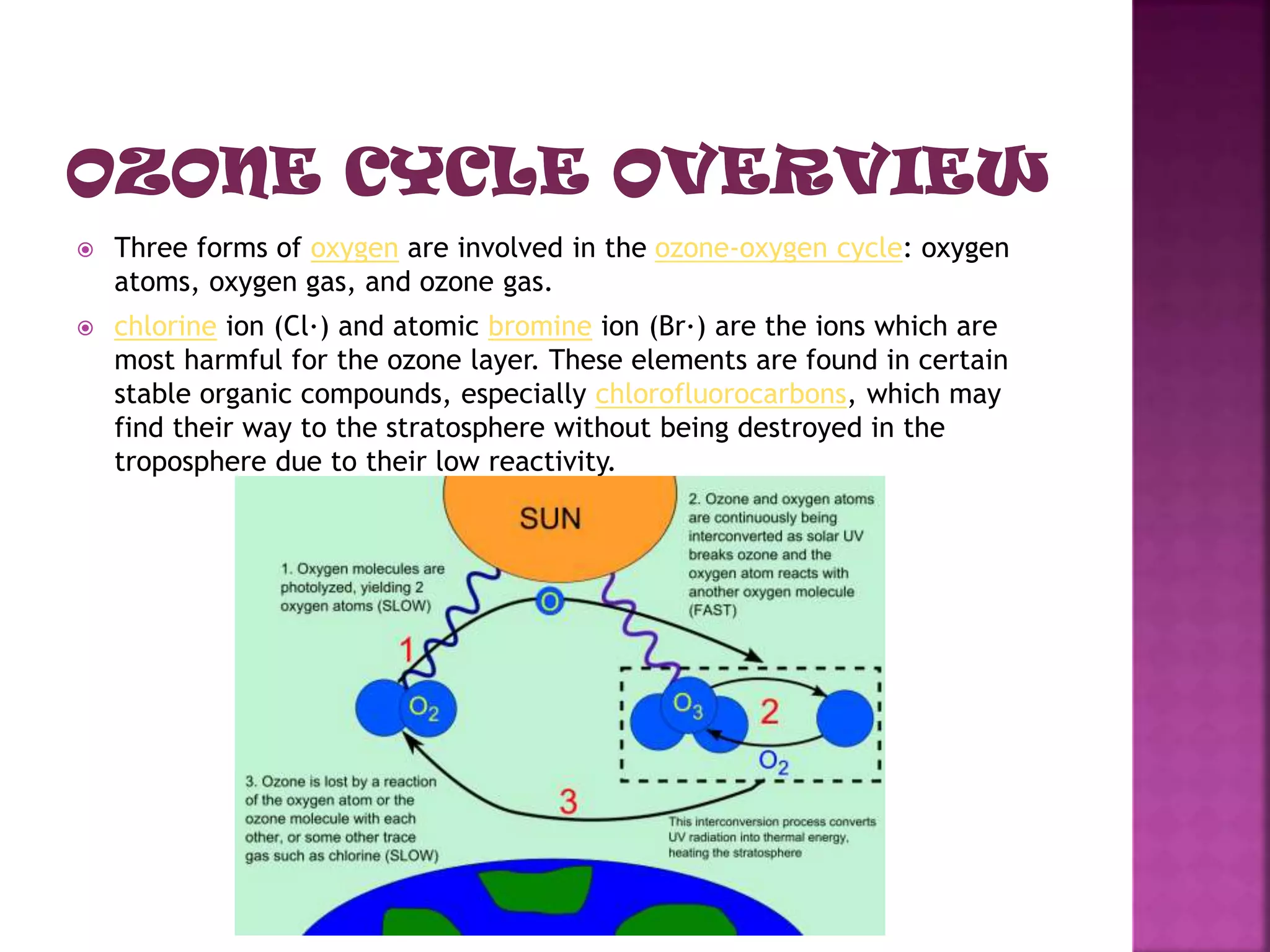
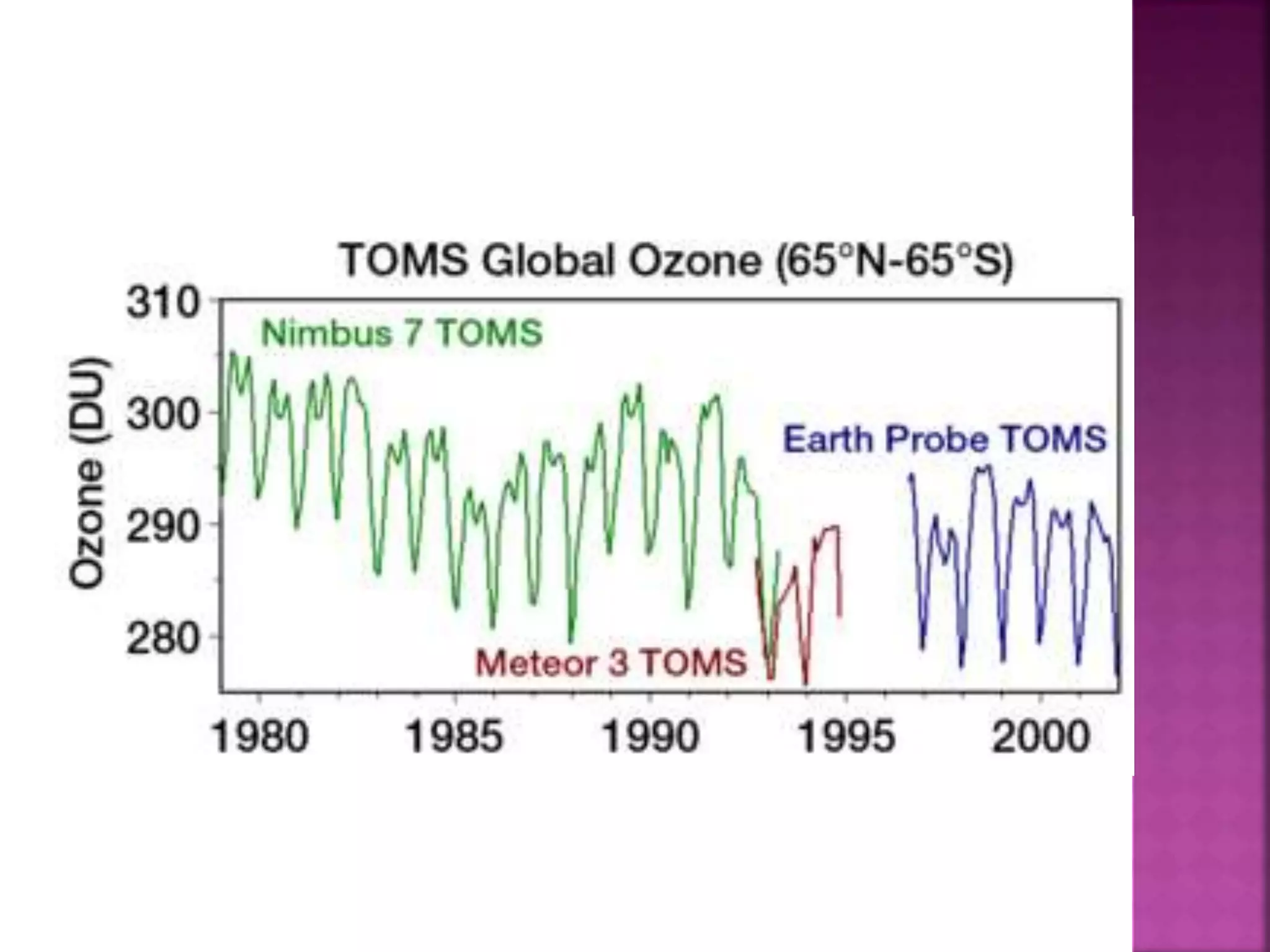
The ozone layer absorbs most UV radiation from the sun. It contains high concentrations of ozone and is mainly located in the lower stratosphere. Chlorofluorocarbons (CFCs) and other halogenated compounds released by human activities are the primary cause of ozone depletion by destroying ozone molecules. This has led to a 4% decline in ozone per decade and the formation of an ozone hole over Antarctica. Increased UV radiation due to ozone depletion poses risks to human health, animals, and crop production. The Montreal Protocol was adopted in 1987 to phase out ozone depleting substances to protect the ozone layer.