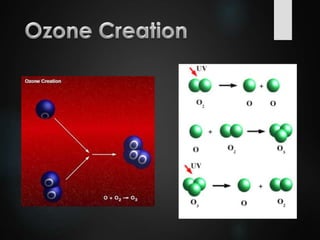
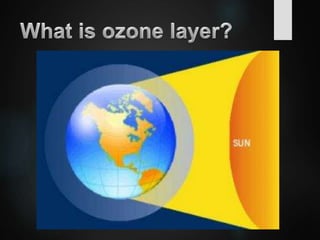
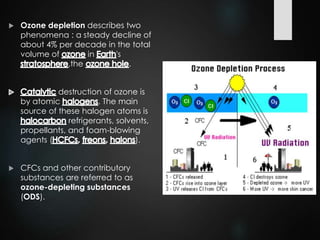
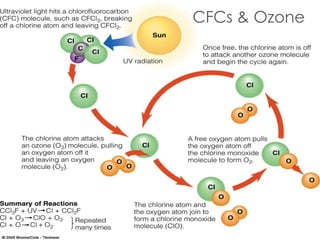
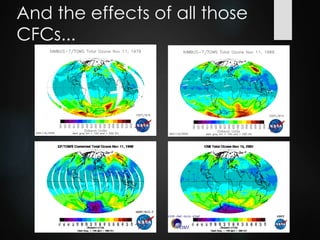
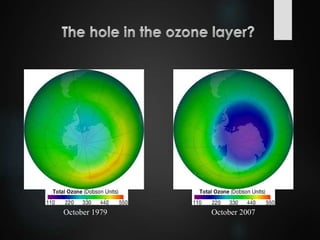
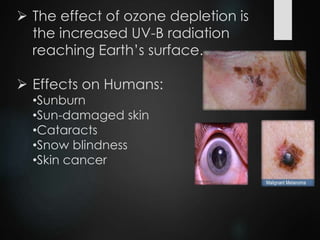
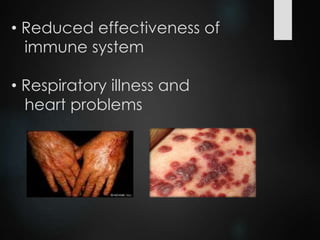
The ozone layer is a thin part of the stratosphere that protects the Earth from harmful UV radiation from the sun. It contains high concentrations of ozone which absorbs most UV radiation. Ozone depletion is caused by ozone-depleting substances like CFCs releasing chlorine and bromine atoms that destroy ozone. This allows more UV radiation to reach the Earth's surface and increases risks of skin cancer, eye cataracts, and harm to plants and animals. International agreements like the Montreal Protocol phased out most ozone-depleting substances, and models predict the ozone layer will recover by 2050-2075.