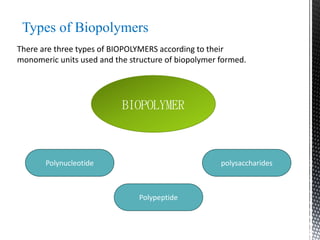
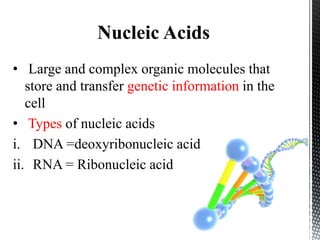
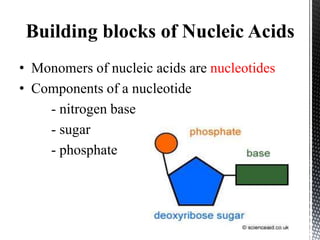
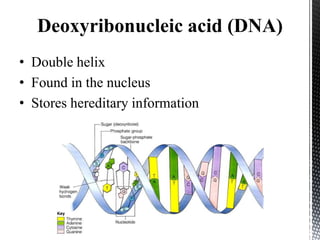
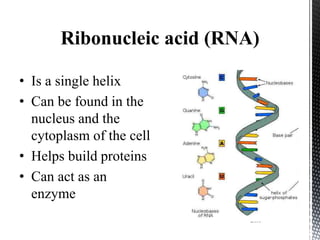
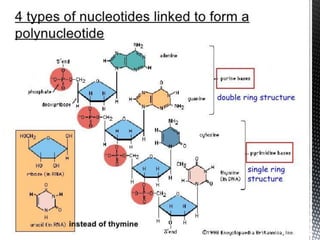
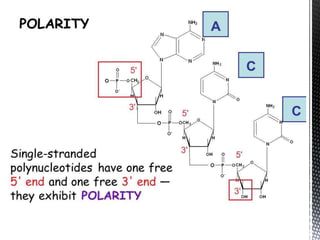
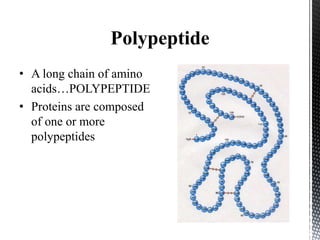
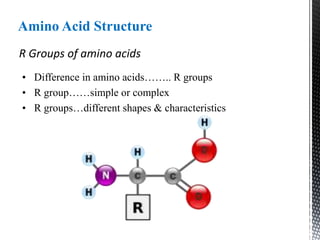
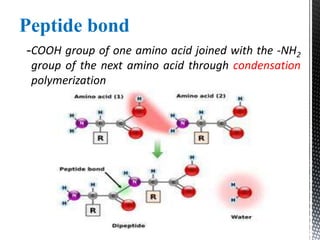
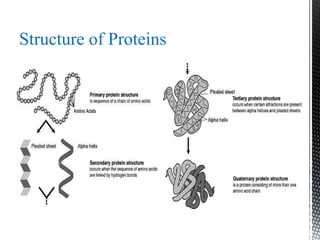
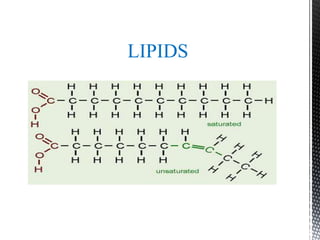
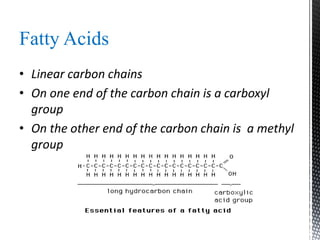
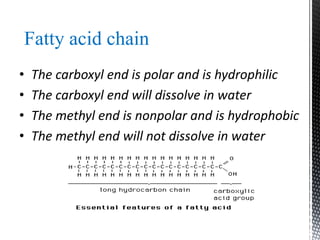
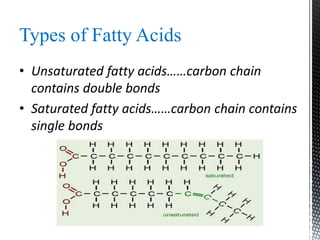
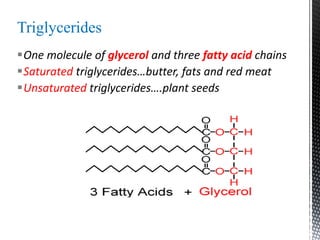
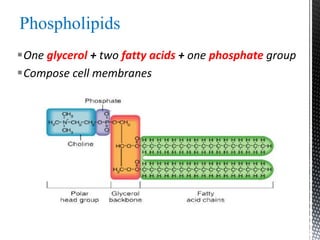
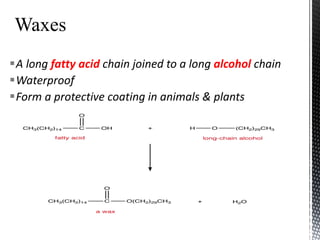
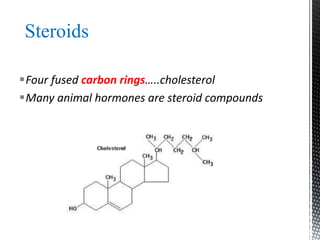
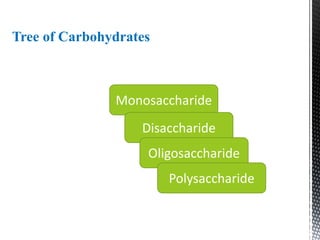
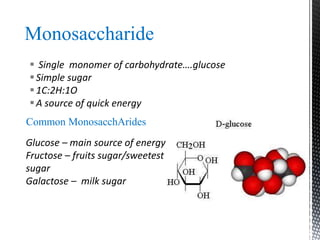
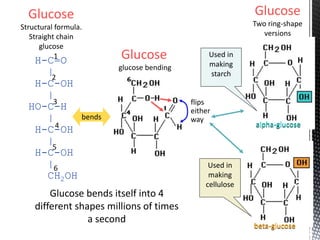
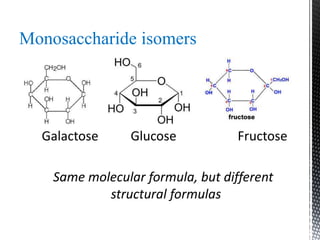
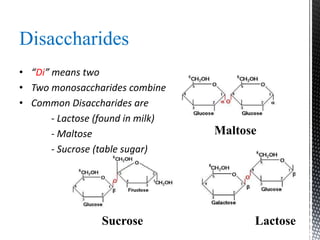
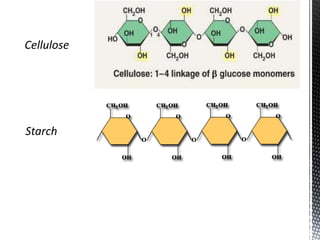
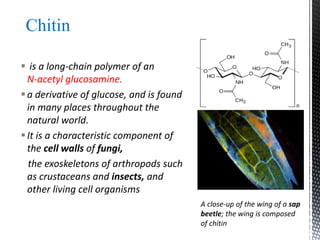
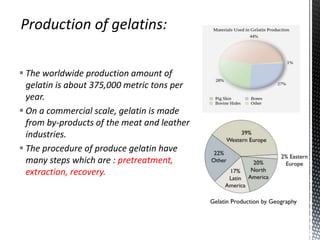
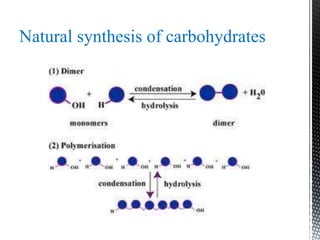
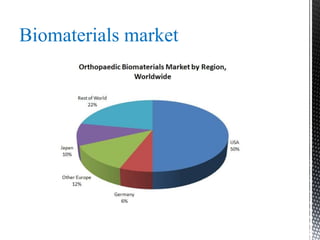
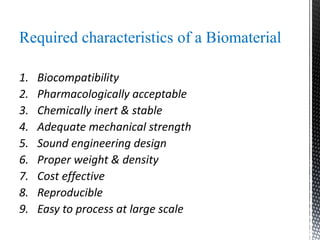
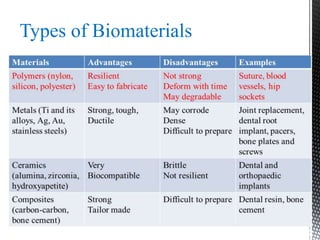
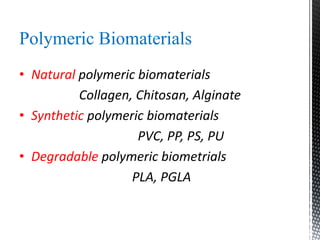
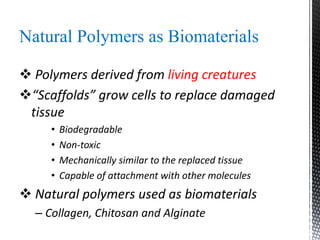
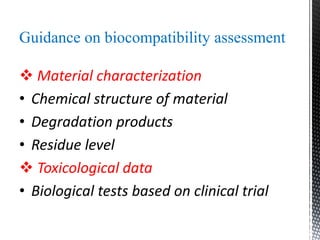
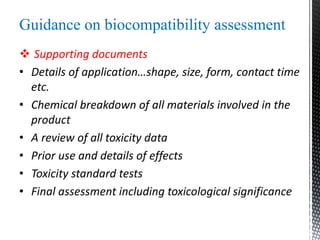
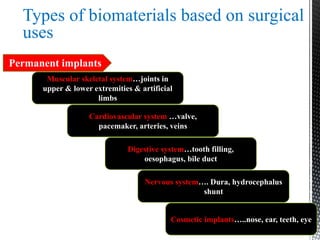
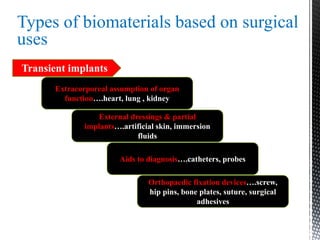
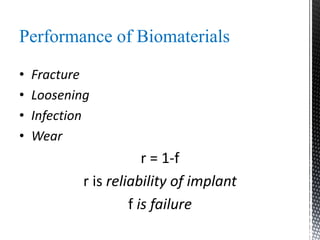
This document provides an overview of different types of biopolymers, including their monomeric units, structures, and examples. The main biopolymers discussed are carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates include monosaccharides like glucose, disaccharides, and polysaccharides. Proteins are composed of amino acid monomers linked through peptide bonds. Lipids include fatty acids, triglycerides, phospholipids, and sterols. Nucleic acids DNA and RNA are made of nucleotides and store genetic information.