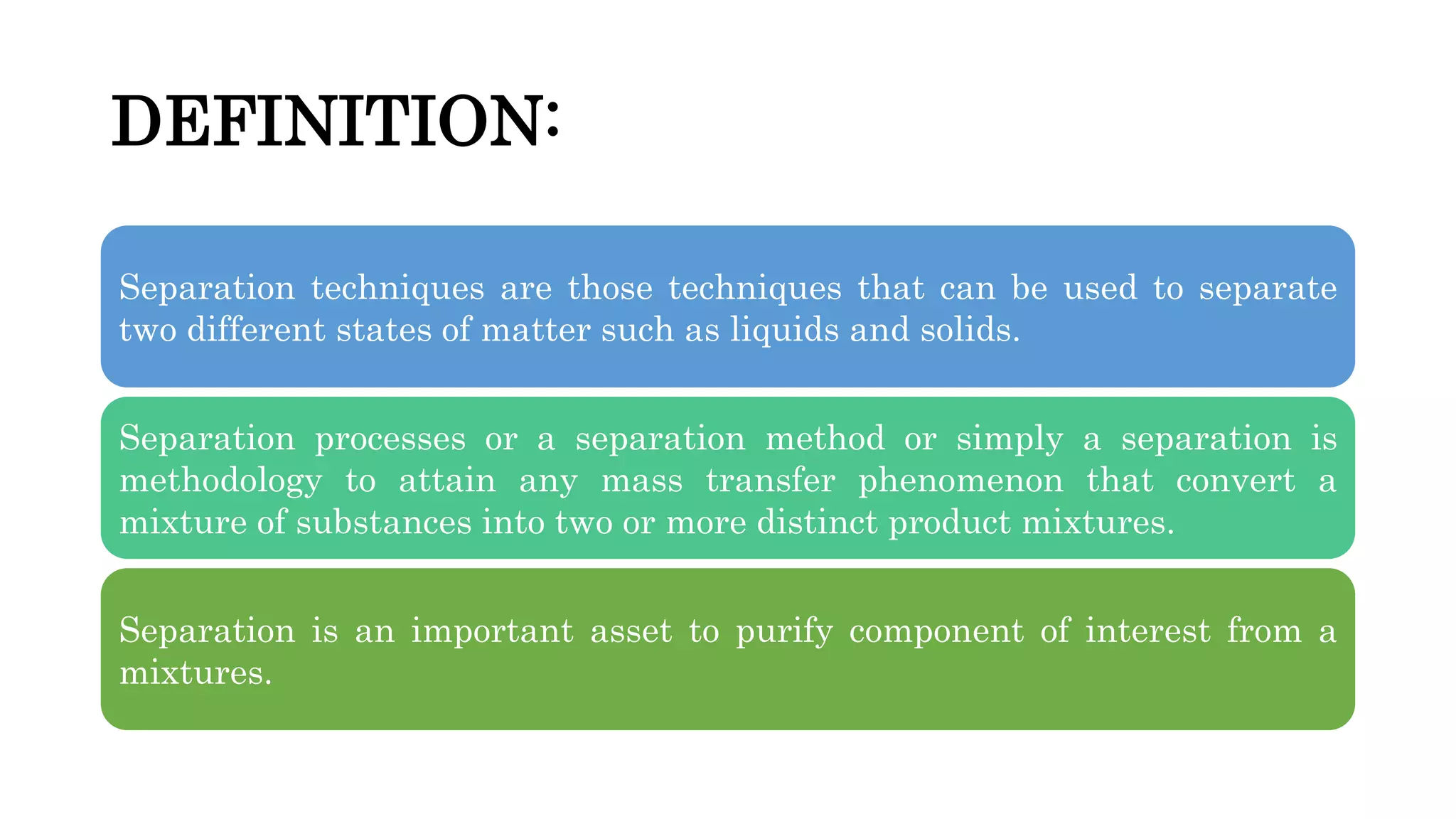
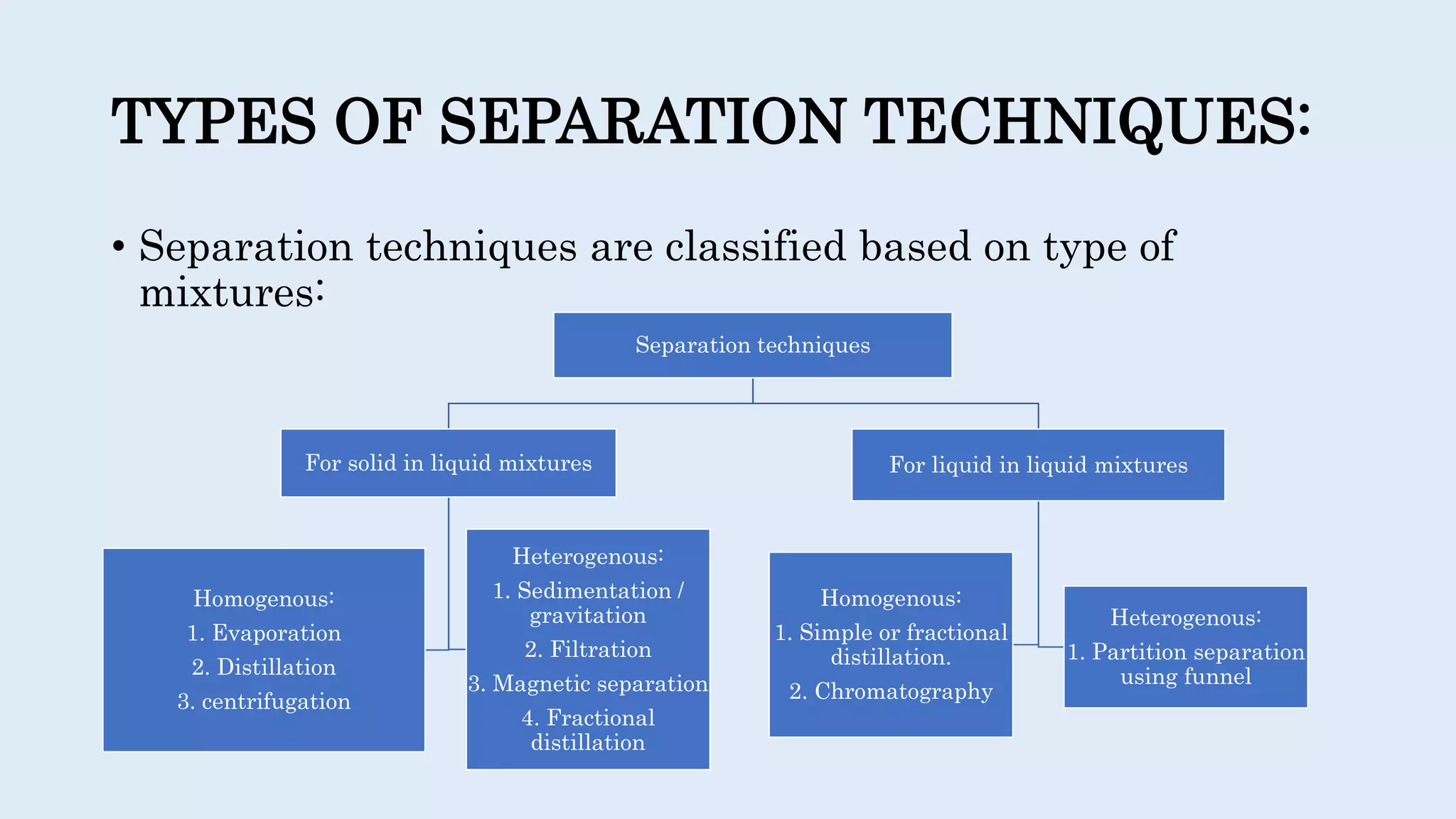
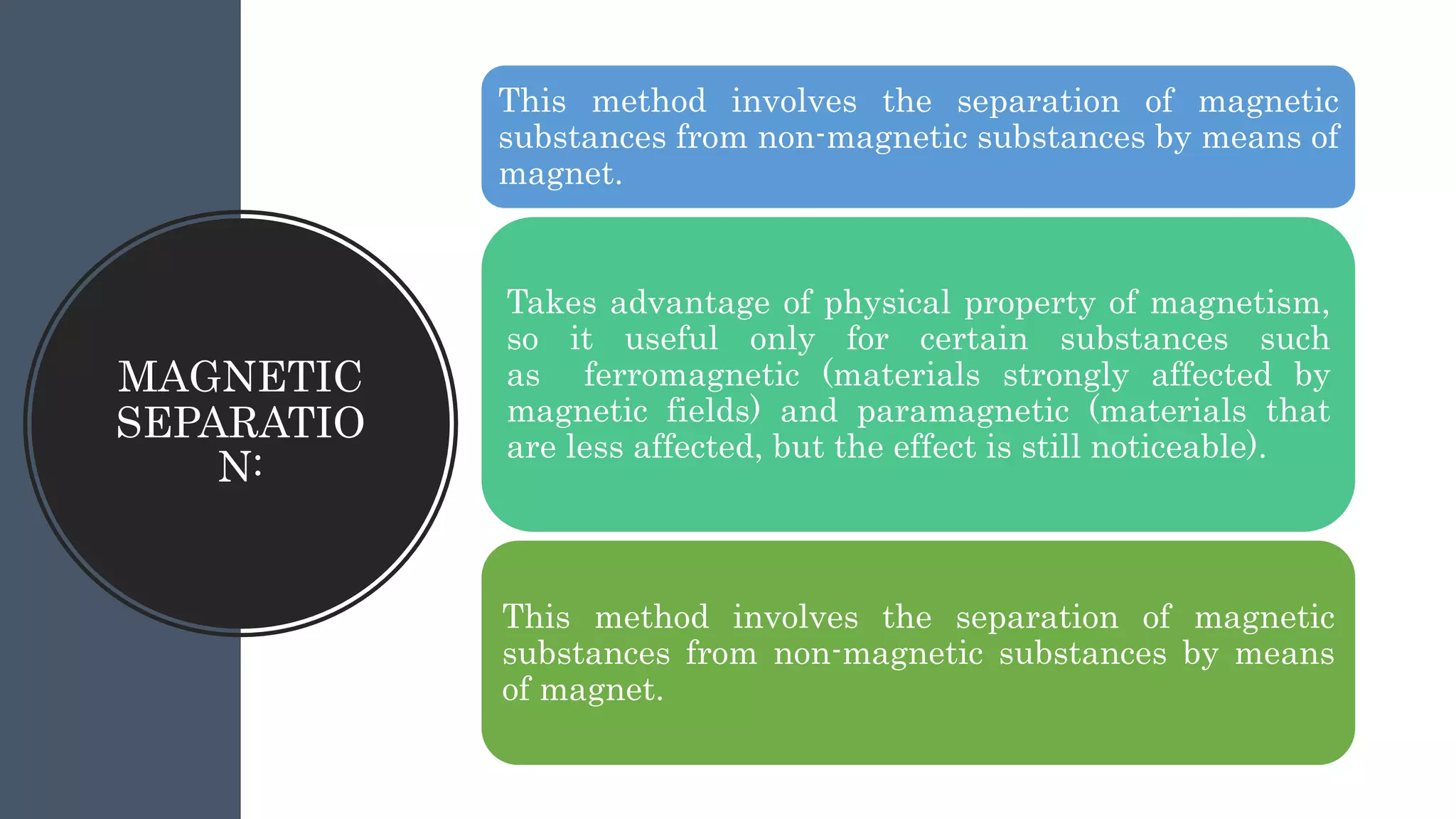
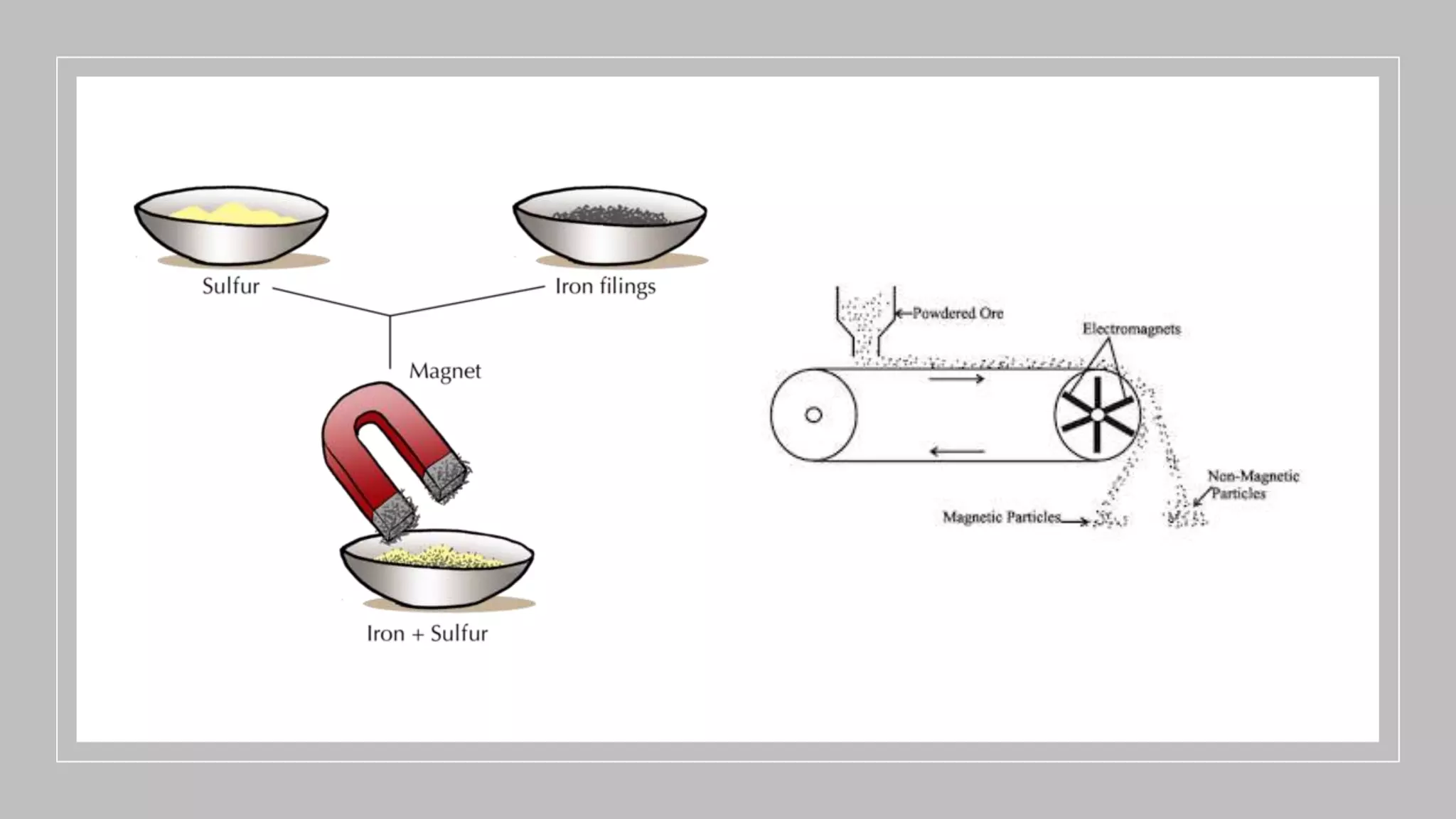
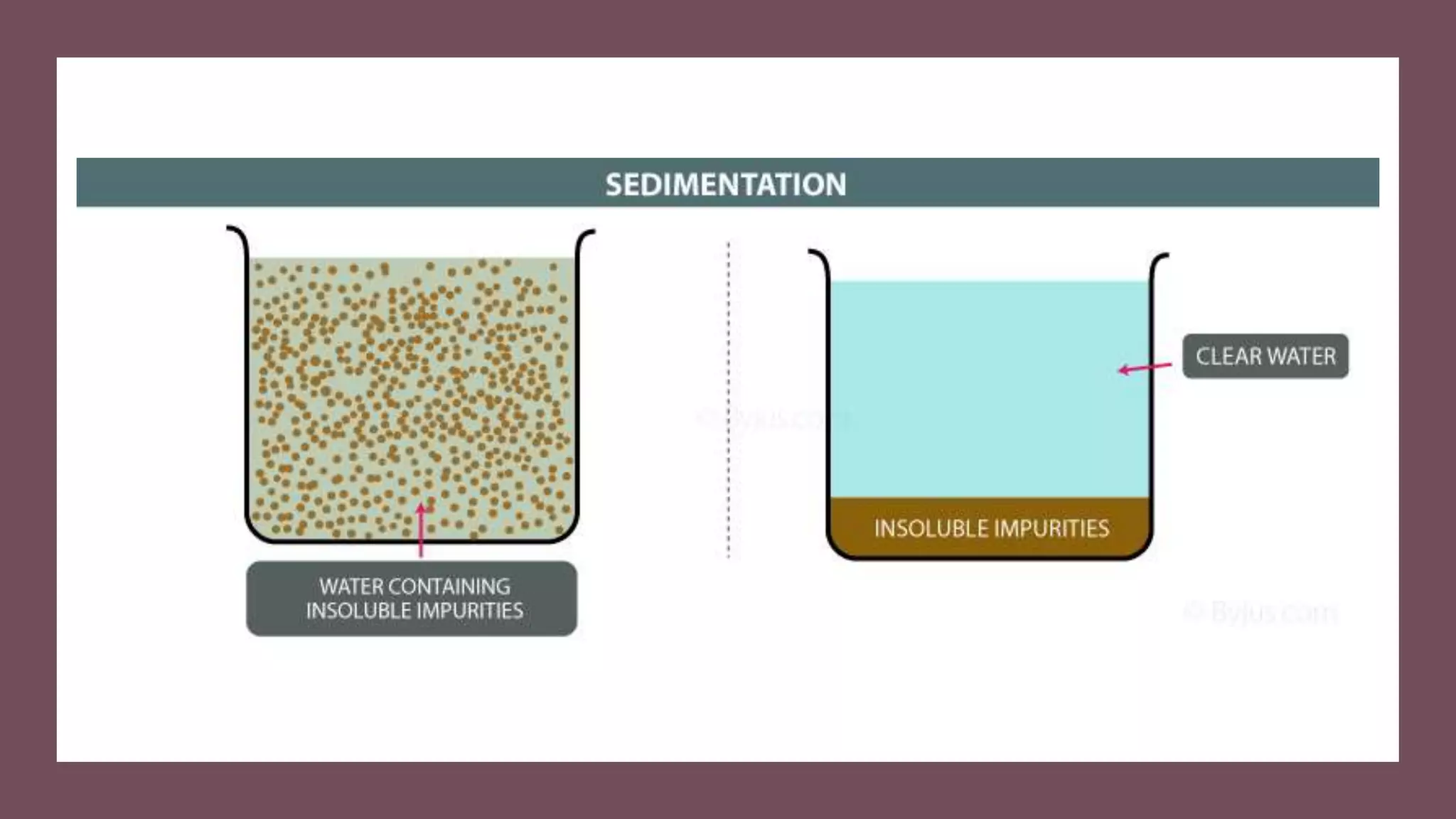
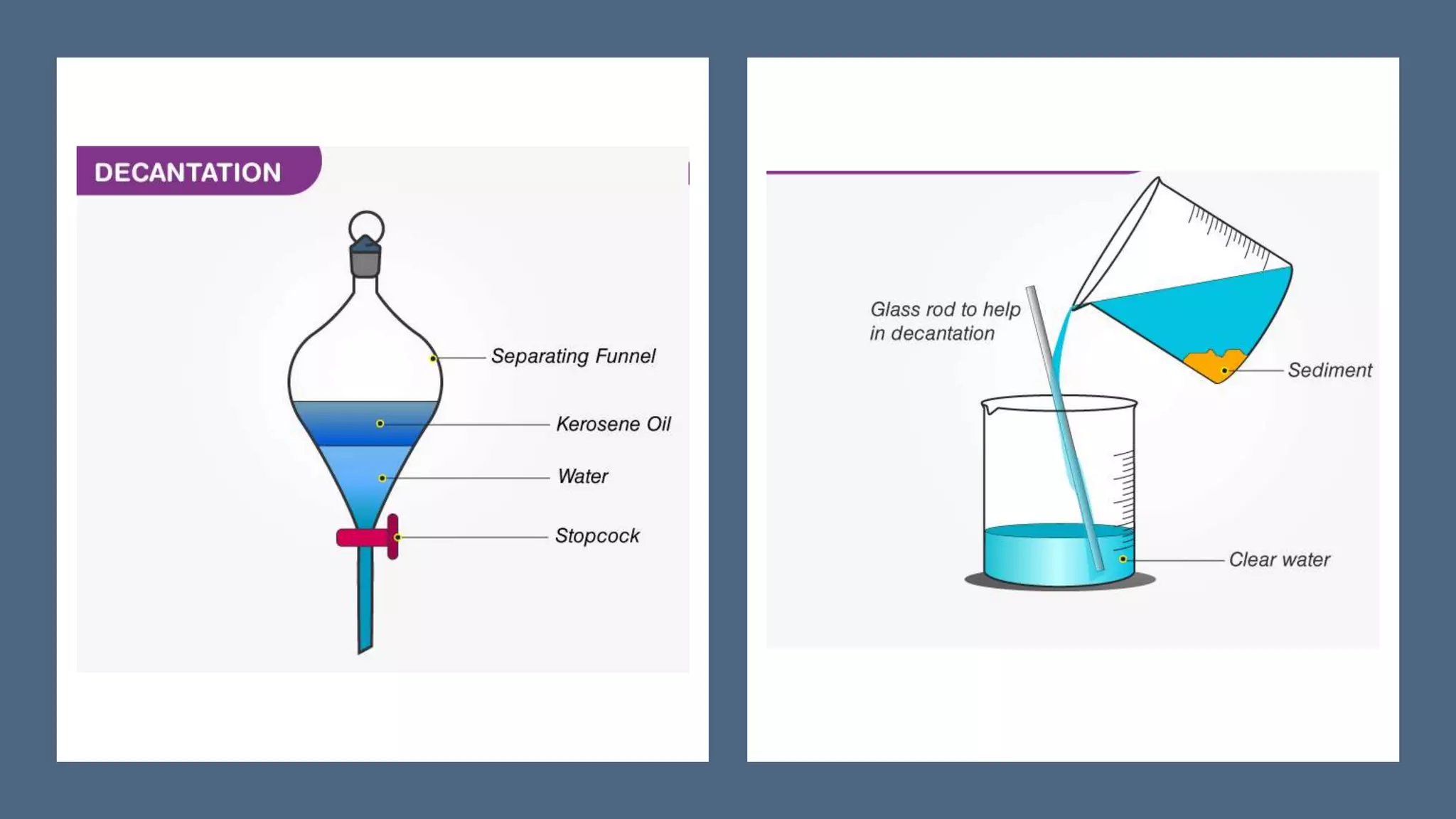
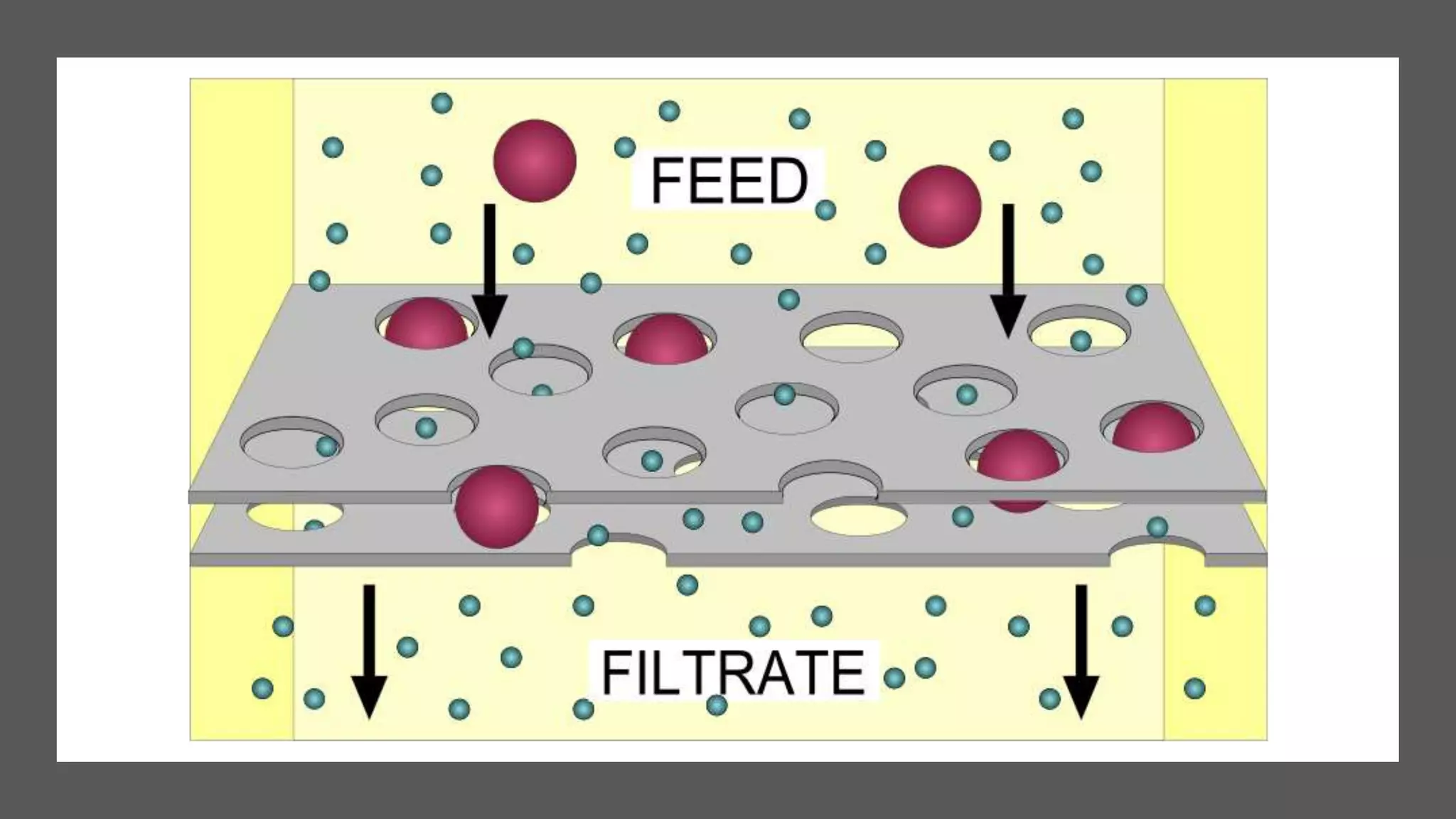
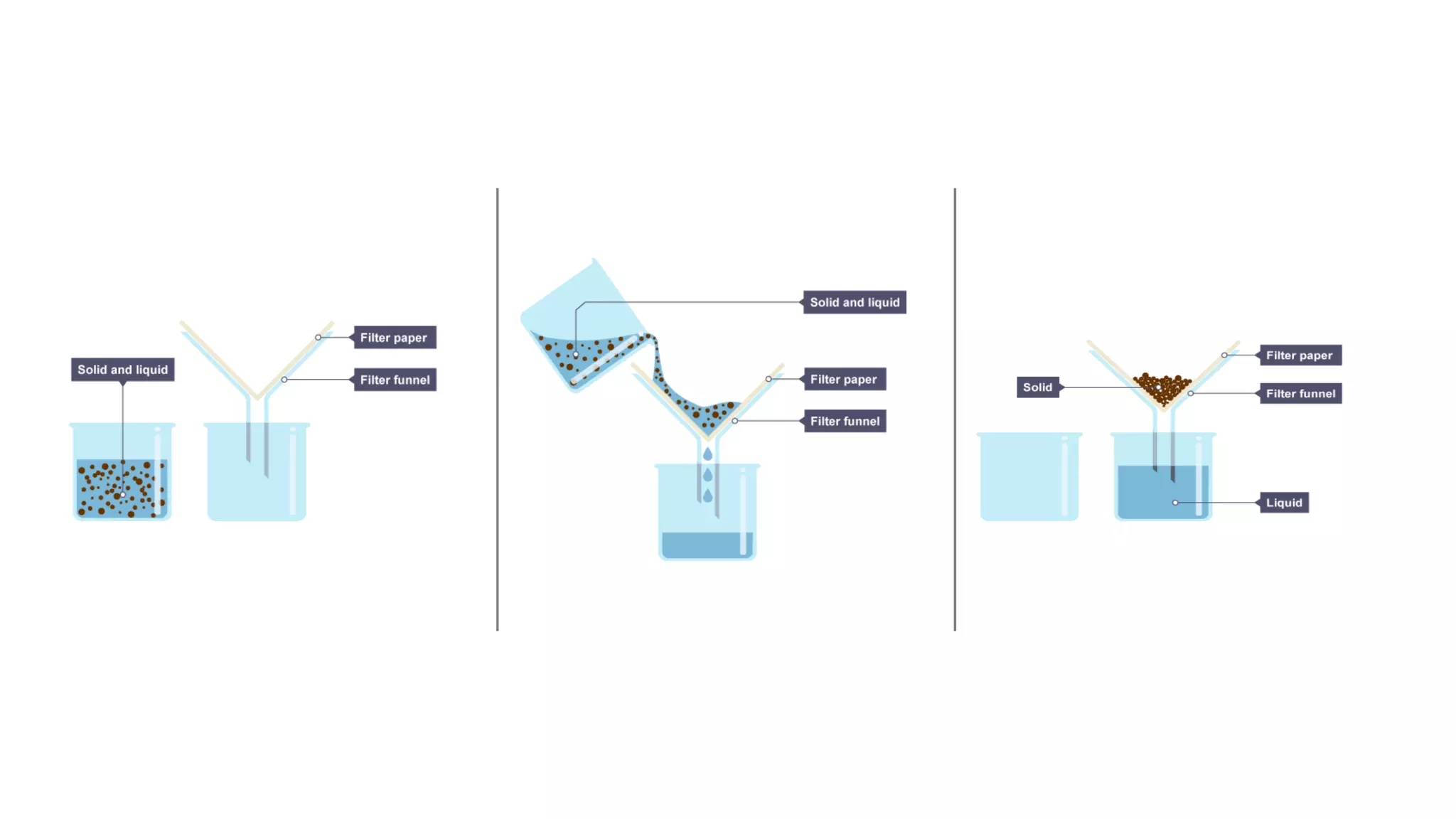
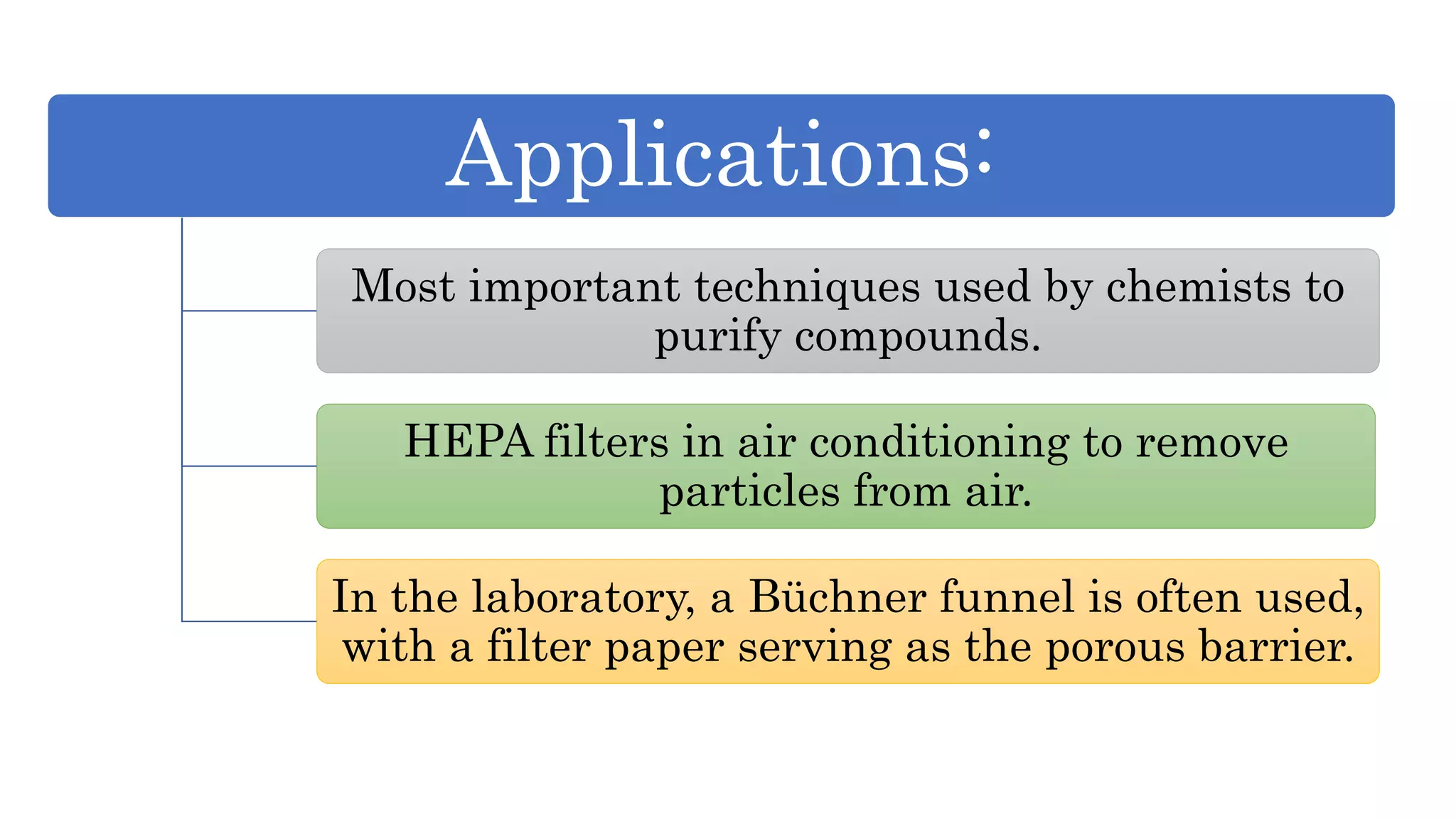
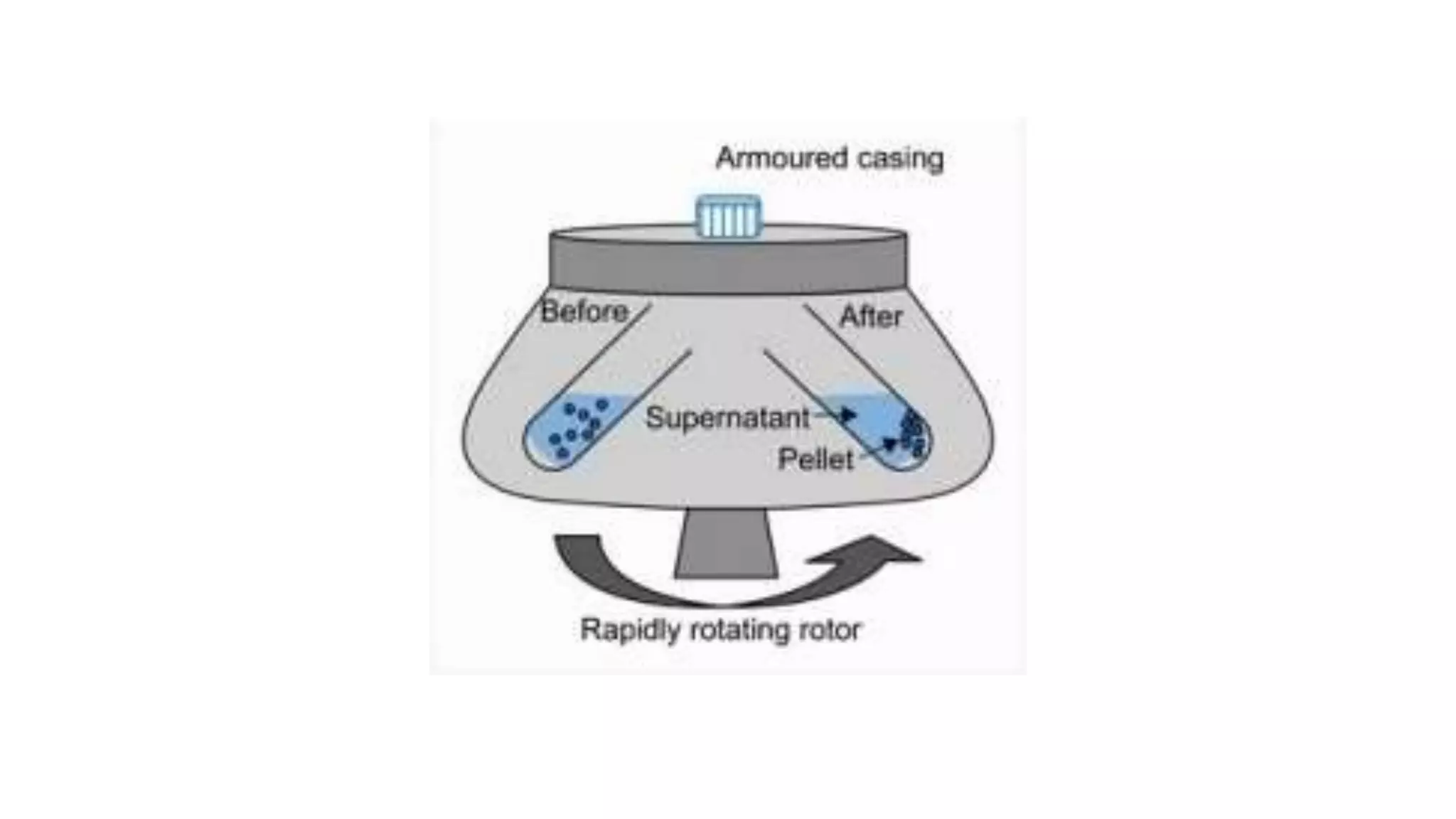
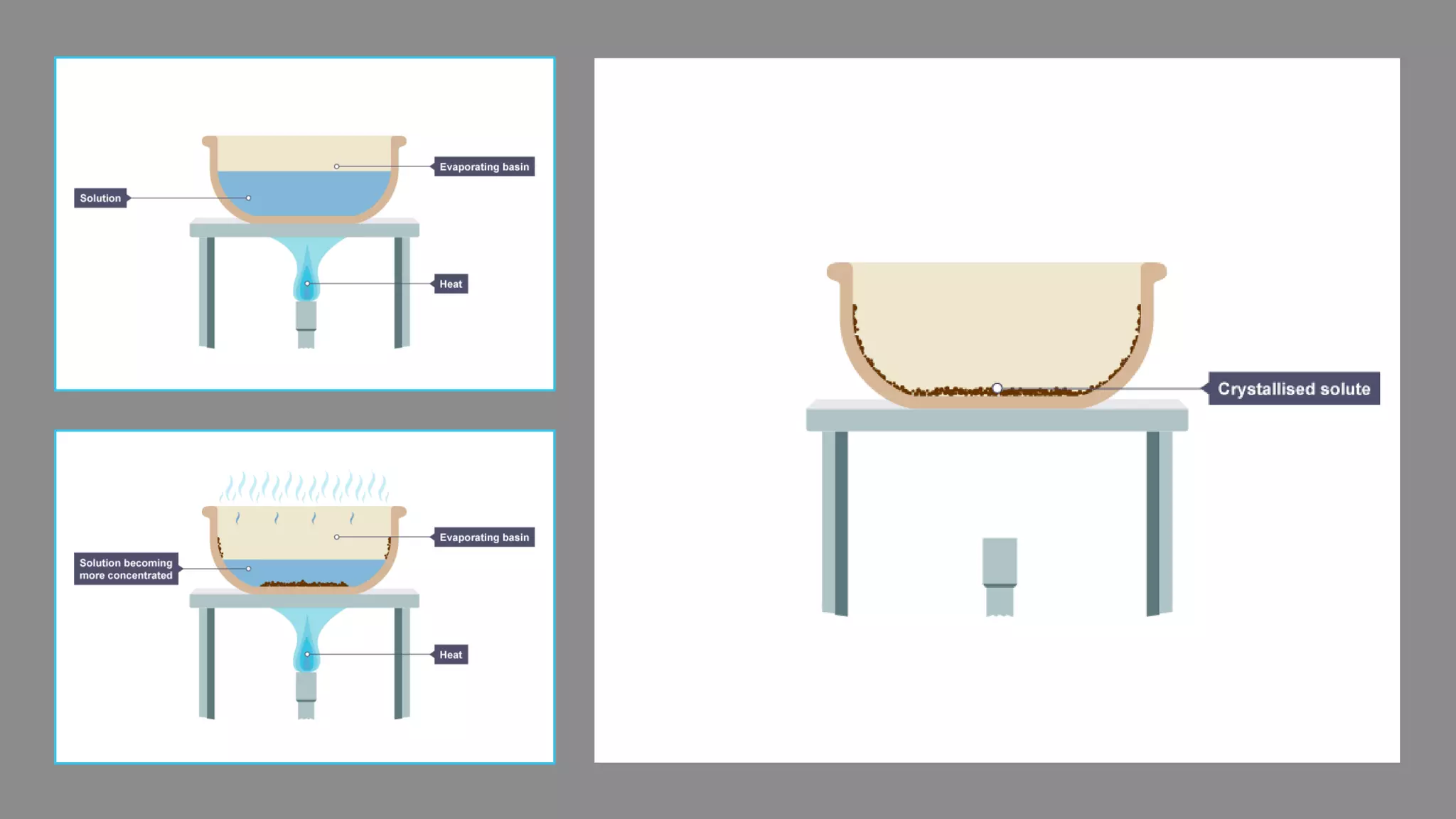
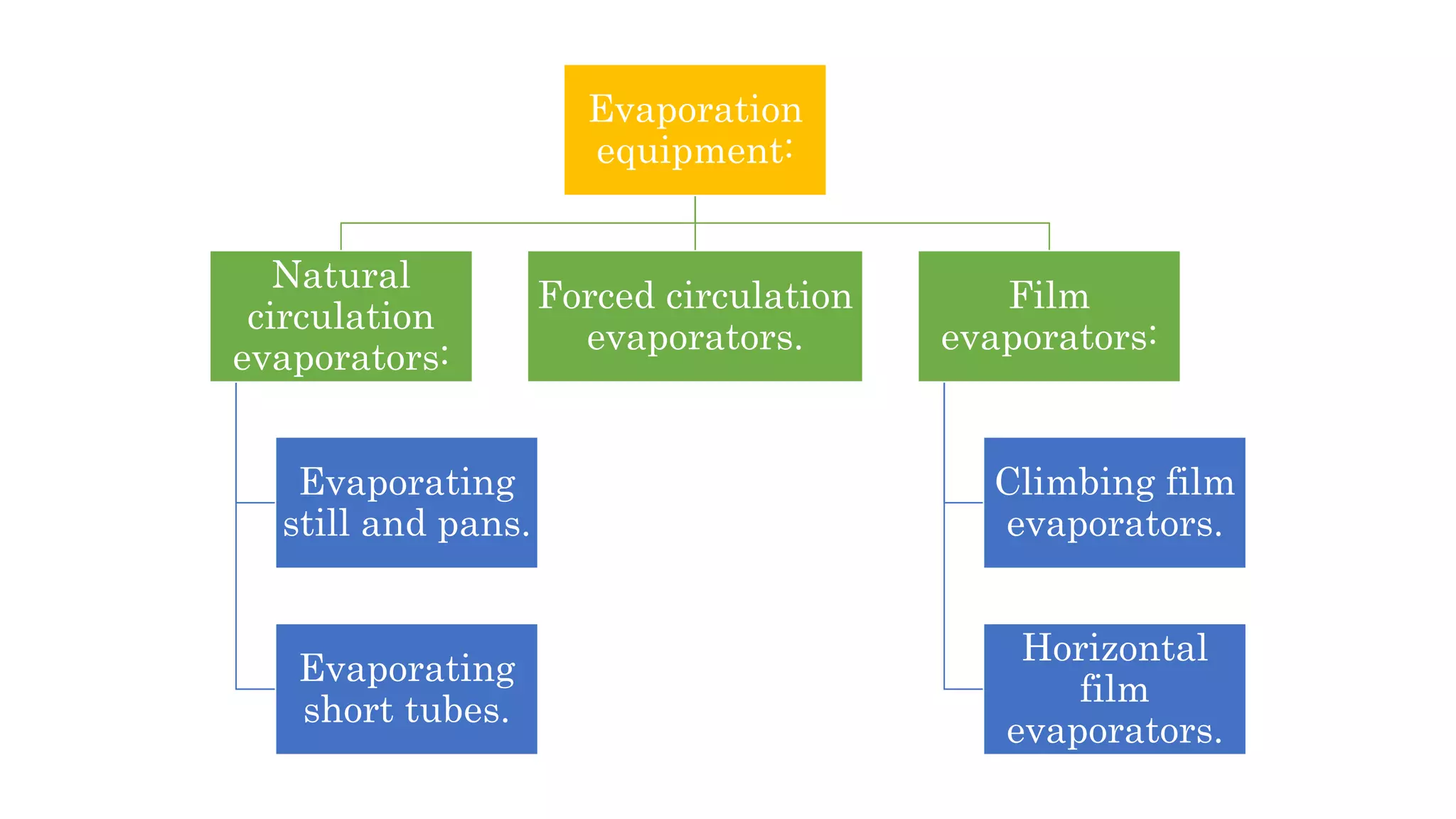
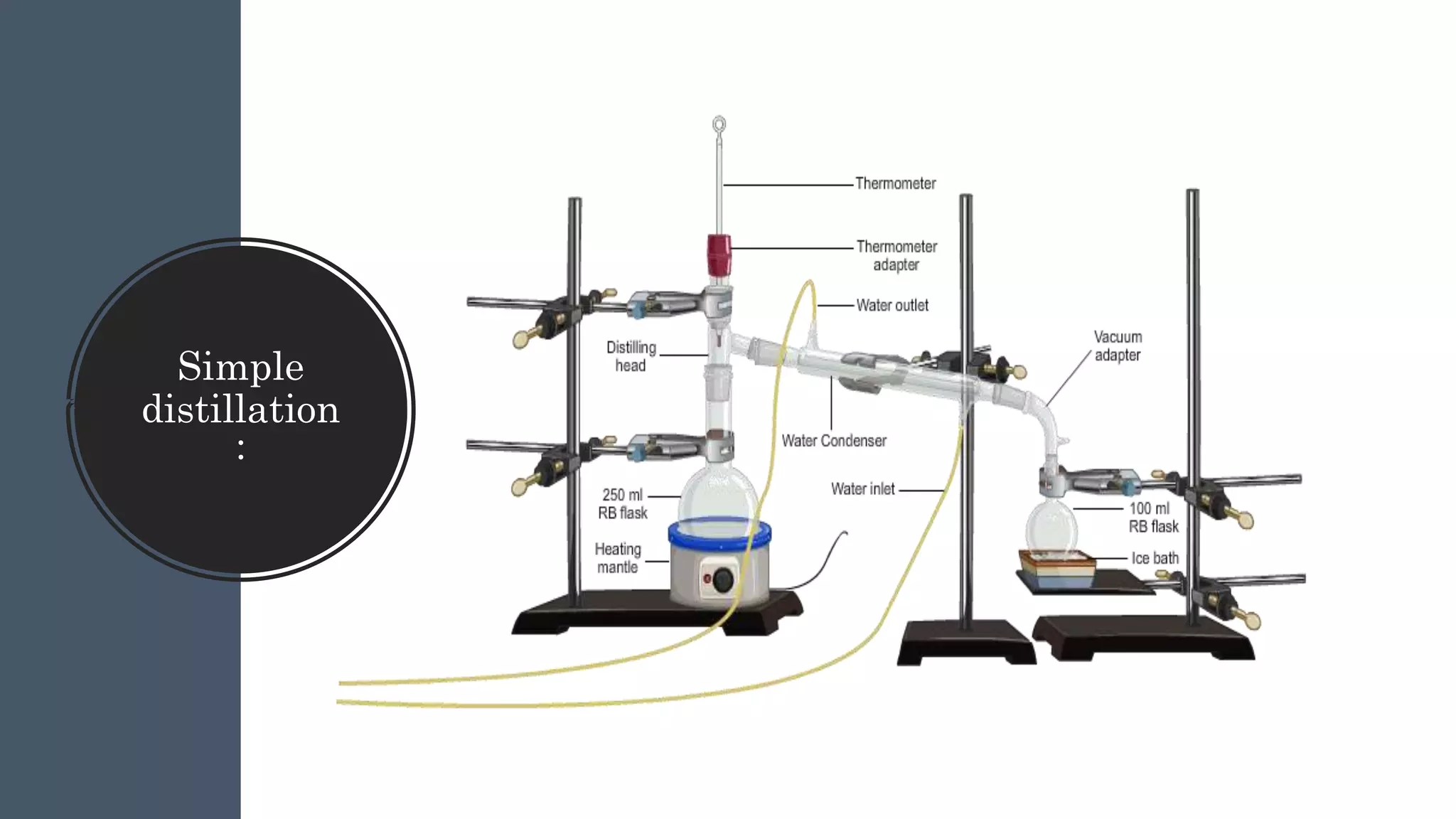
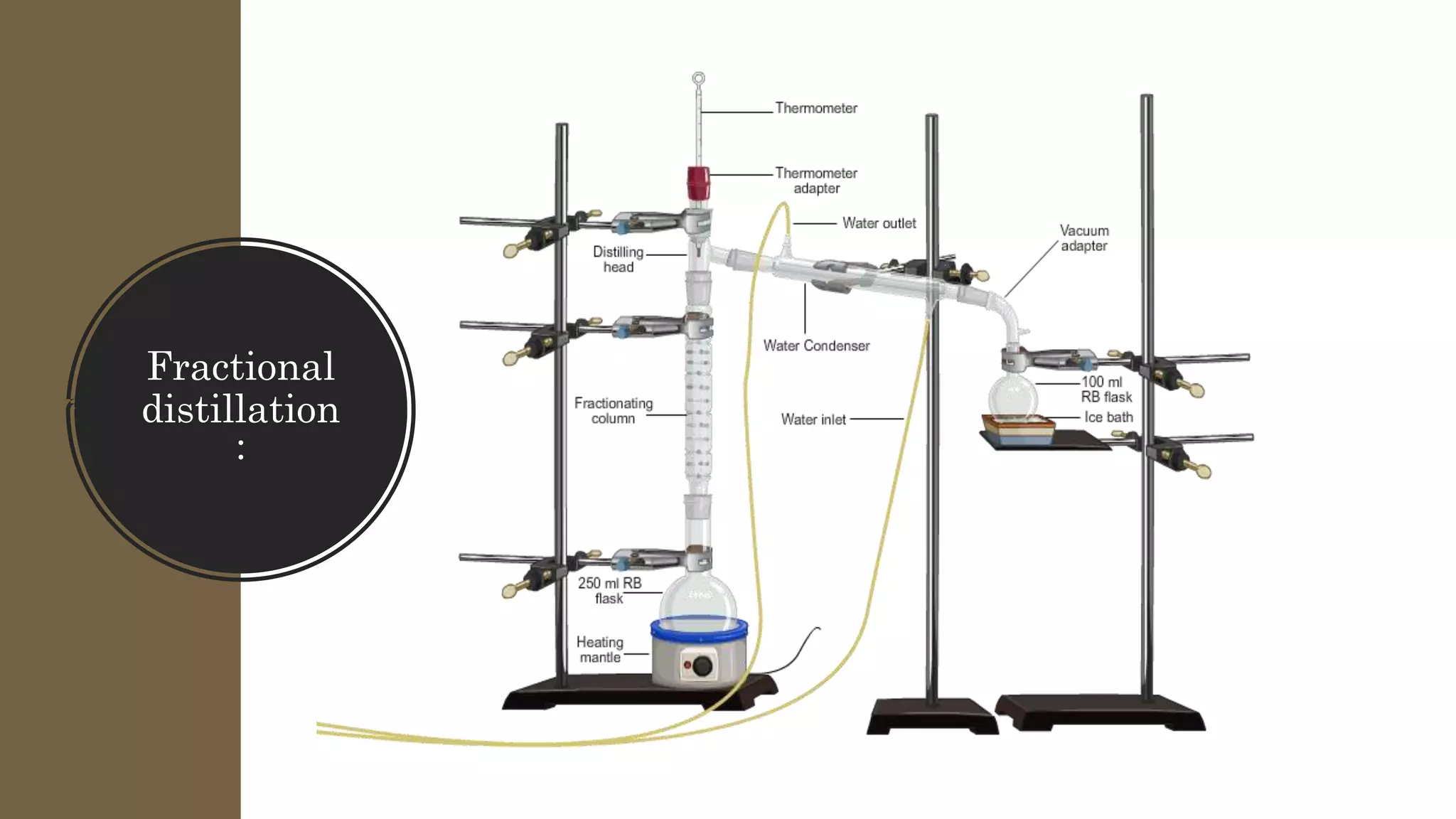
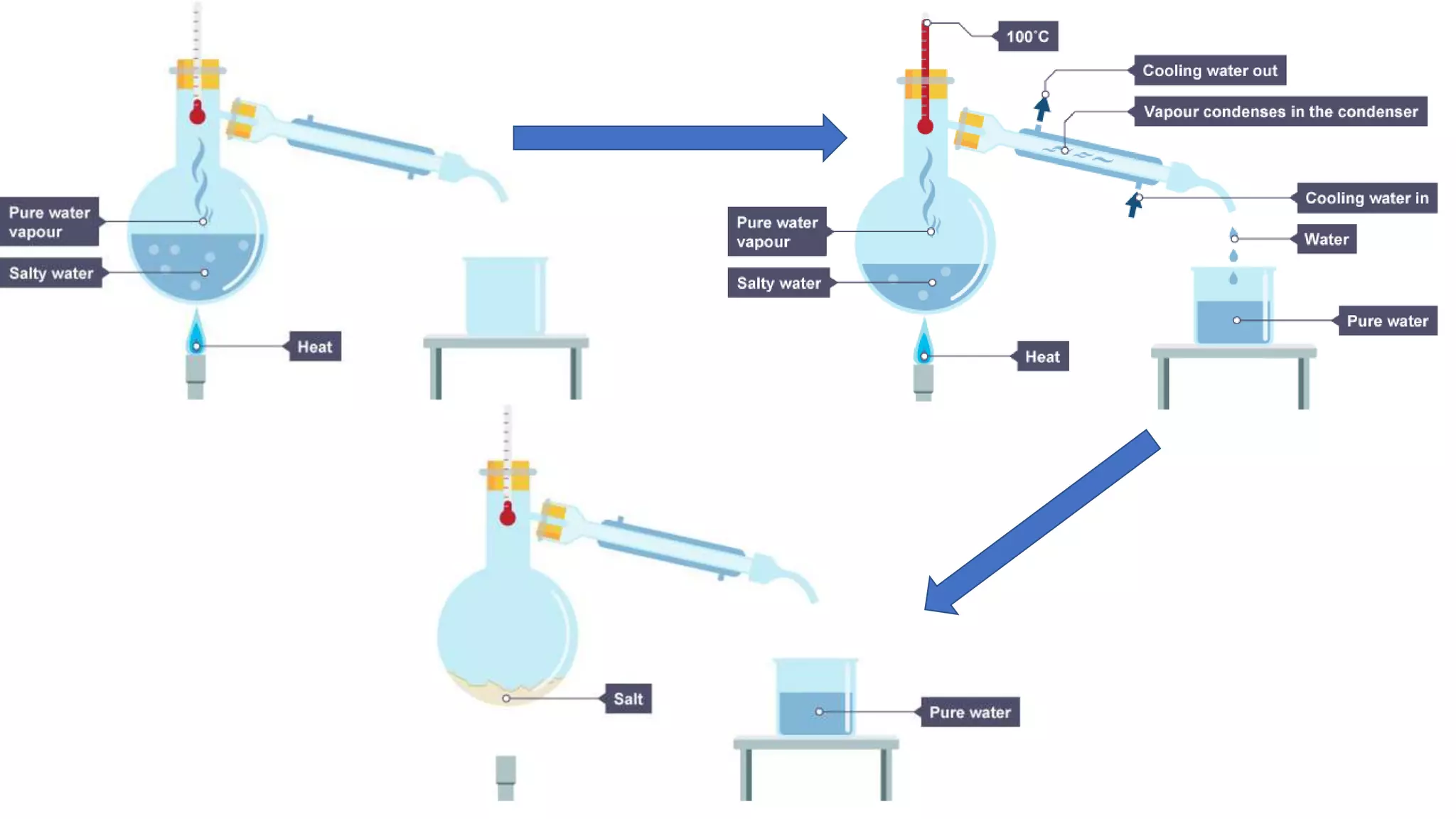
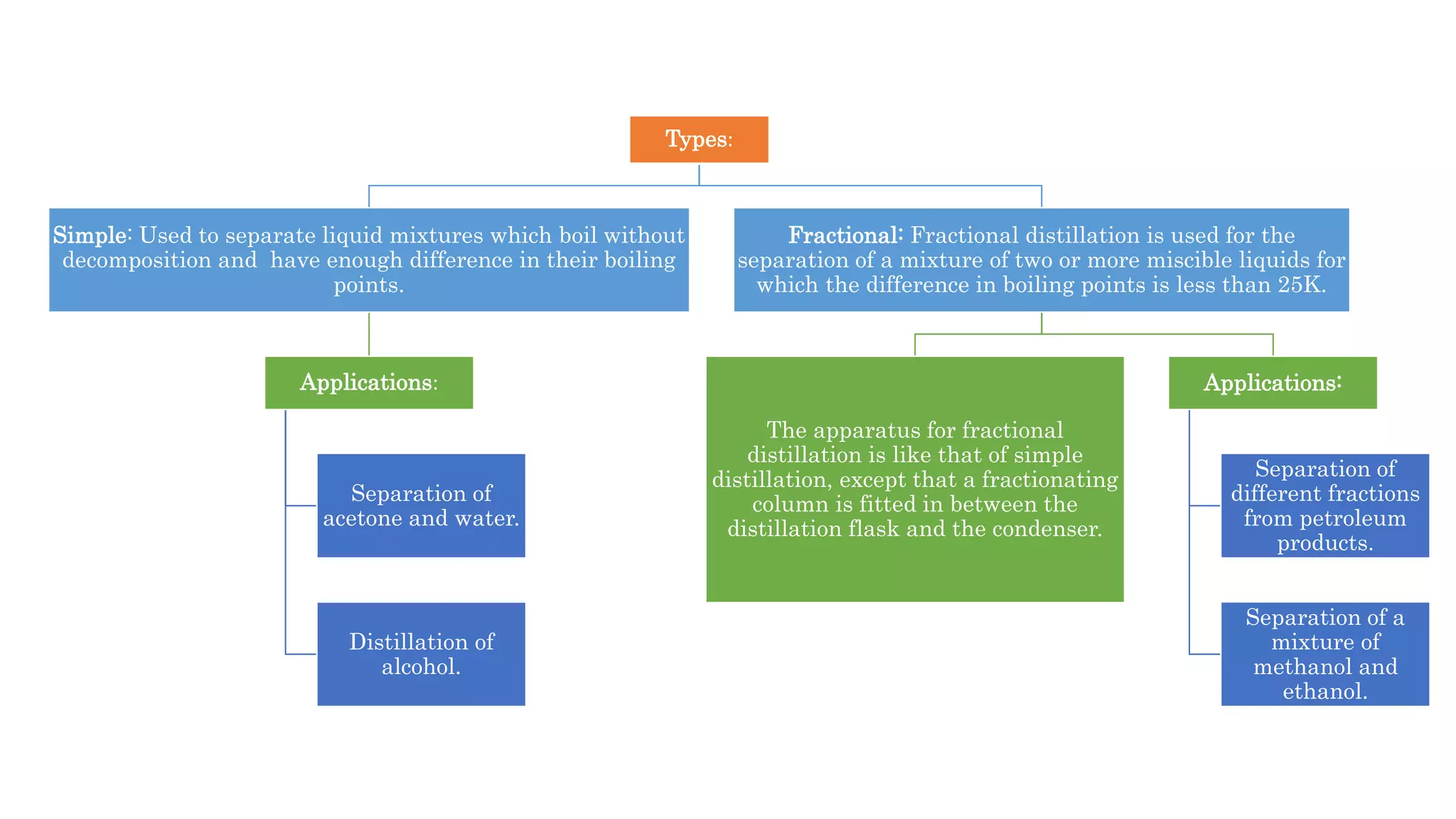
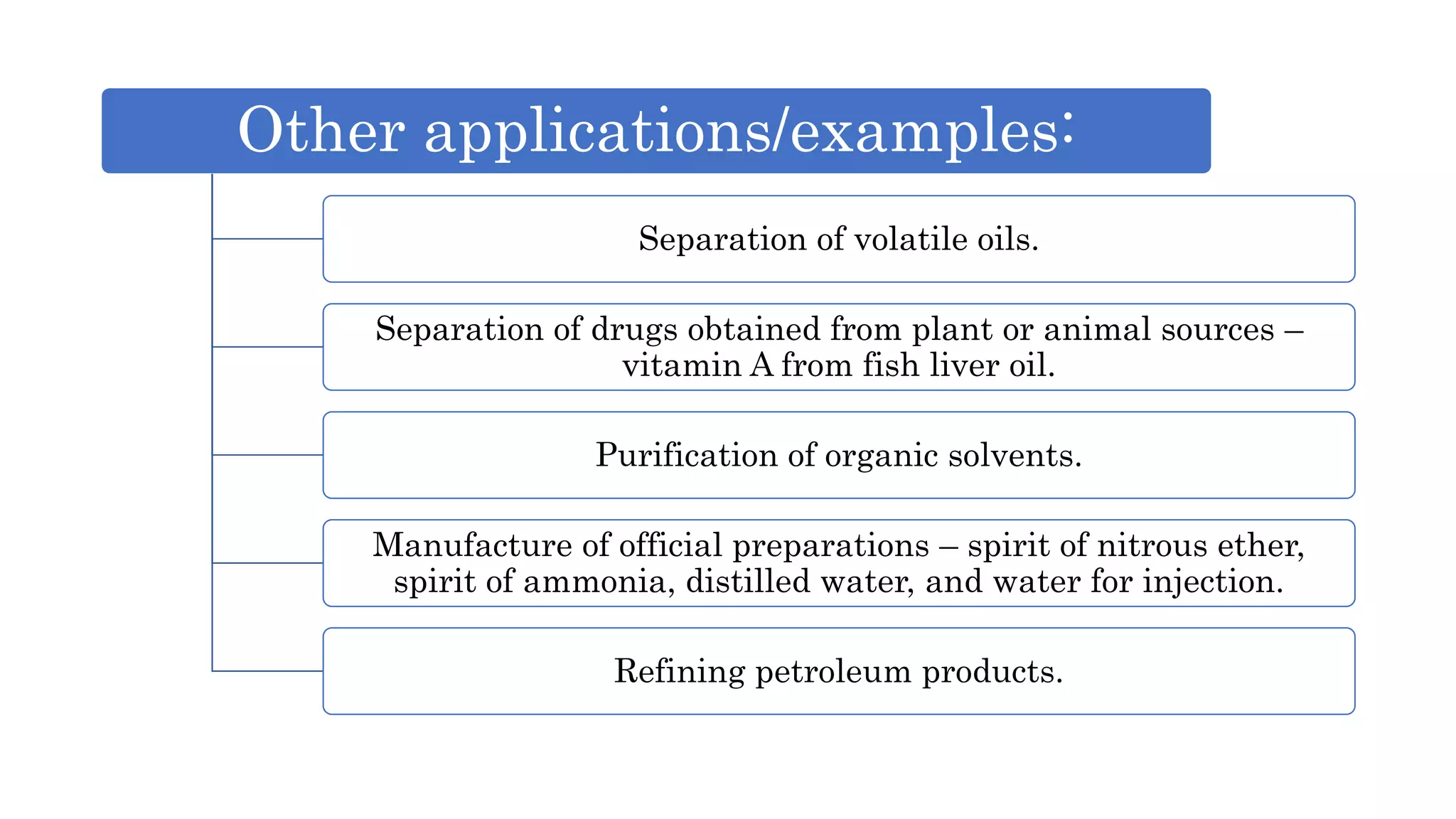
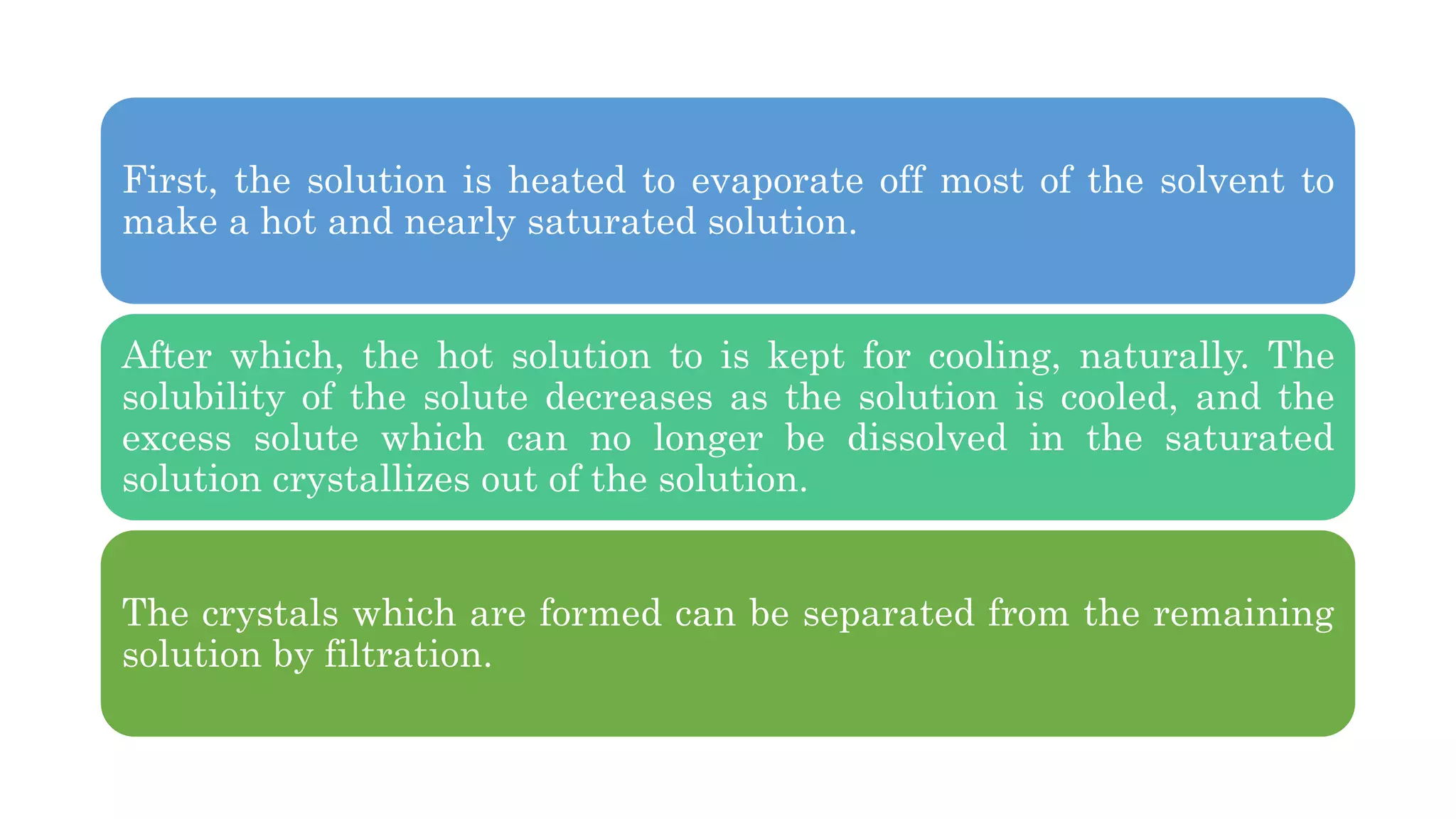
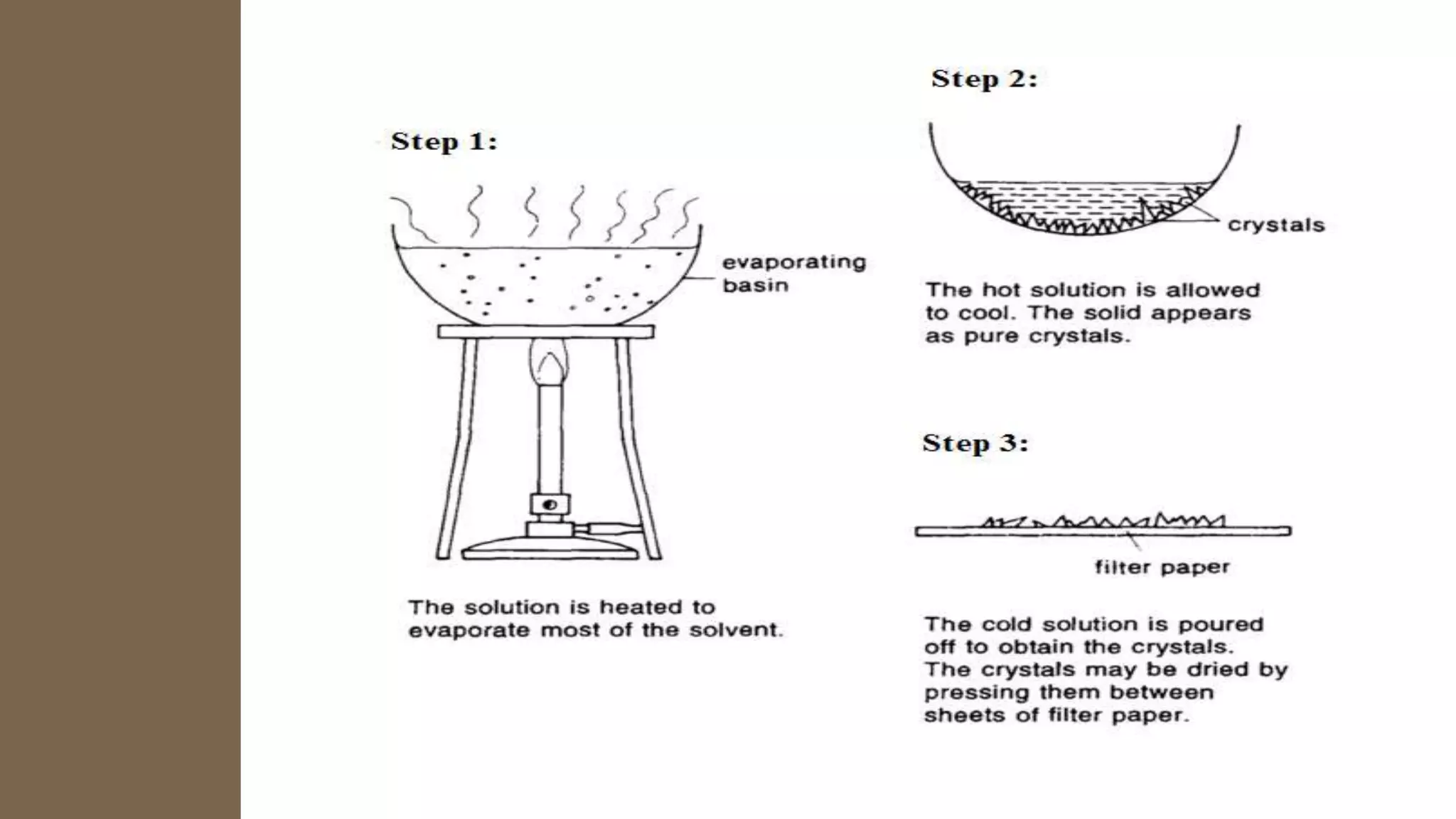
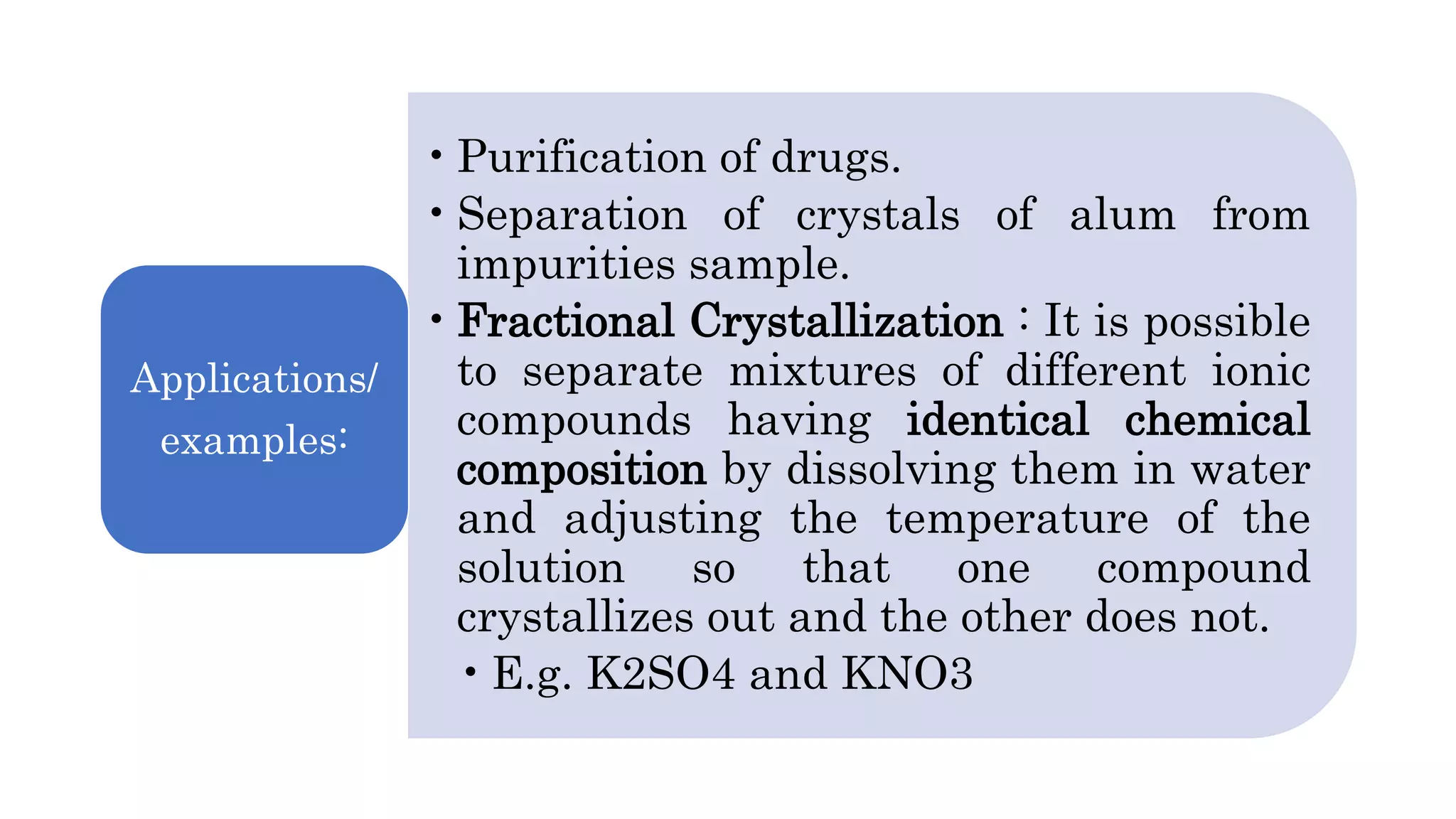
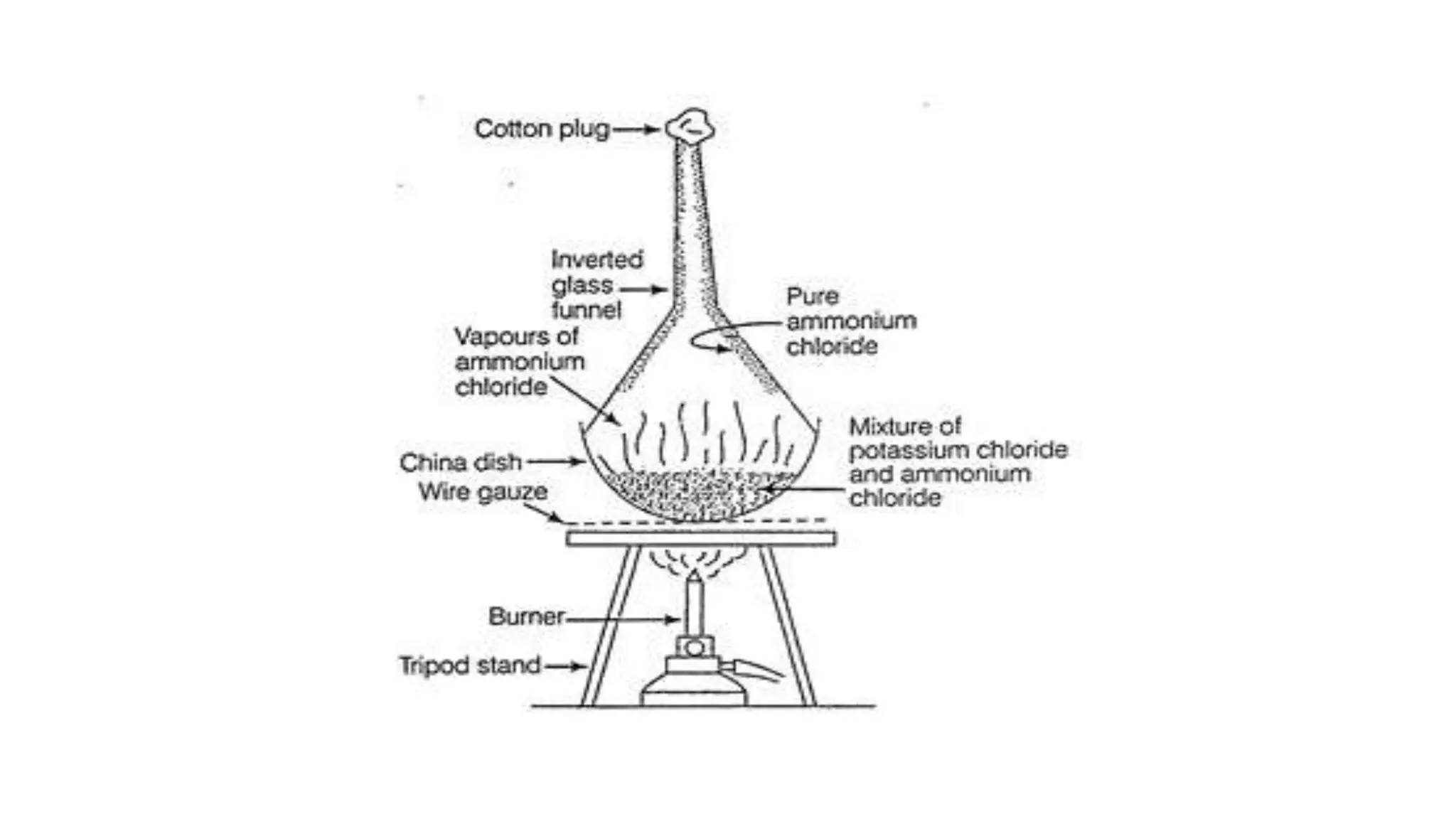
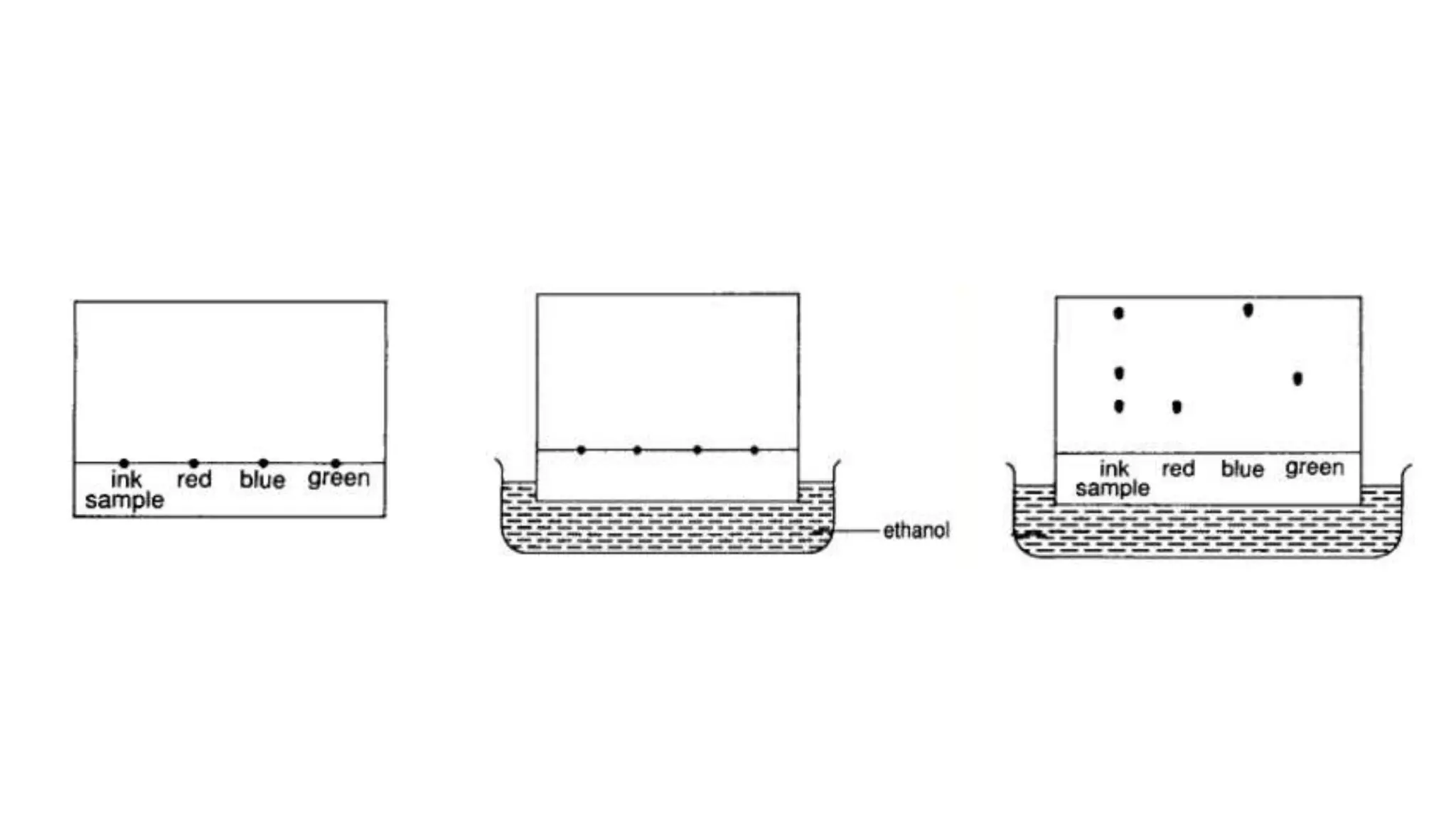
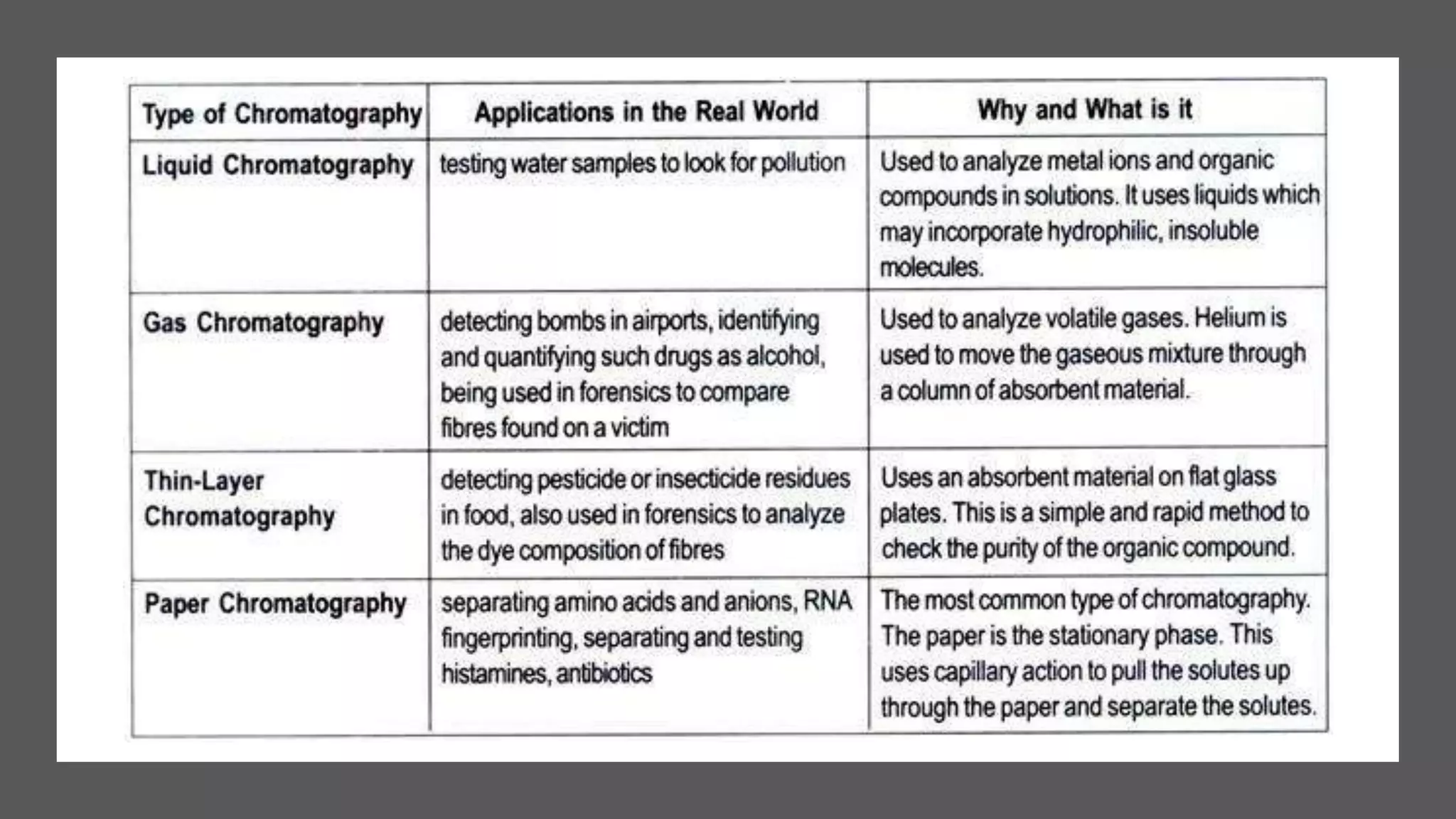
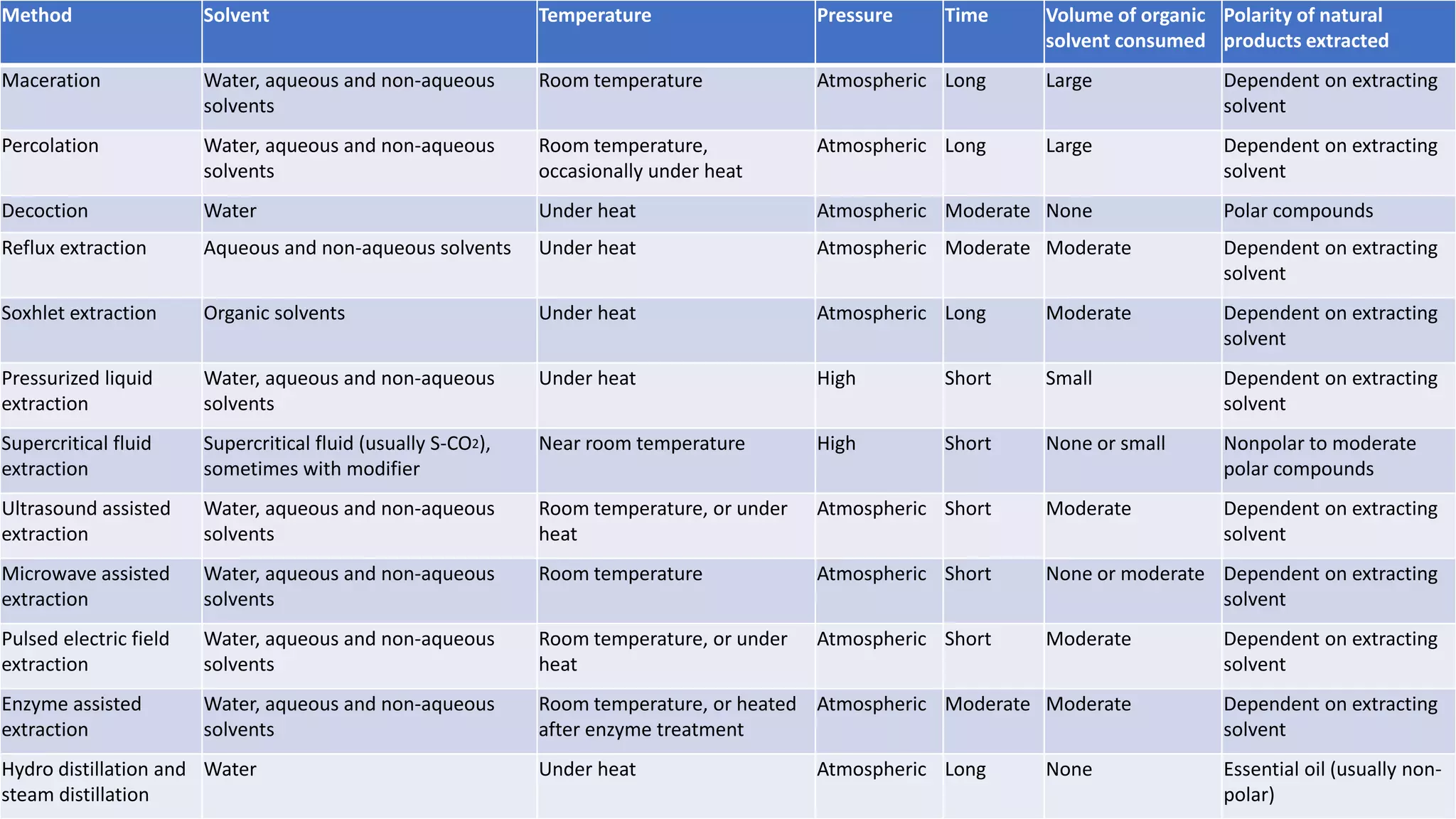
Separation techniques are methodologies used to separate different states of matter, such as liquids and solids, to purify substances or remove unwanted particles. Various methods, including evaporation, distillation, filtration, and chromatography, are employed depending on the type of mixture and the desired result. Applications include waste management, pharmaceuticals, and clinical therapies, utilizing specific properties such as density or boiling point for effective separation.