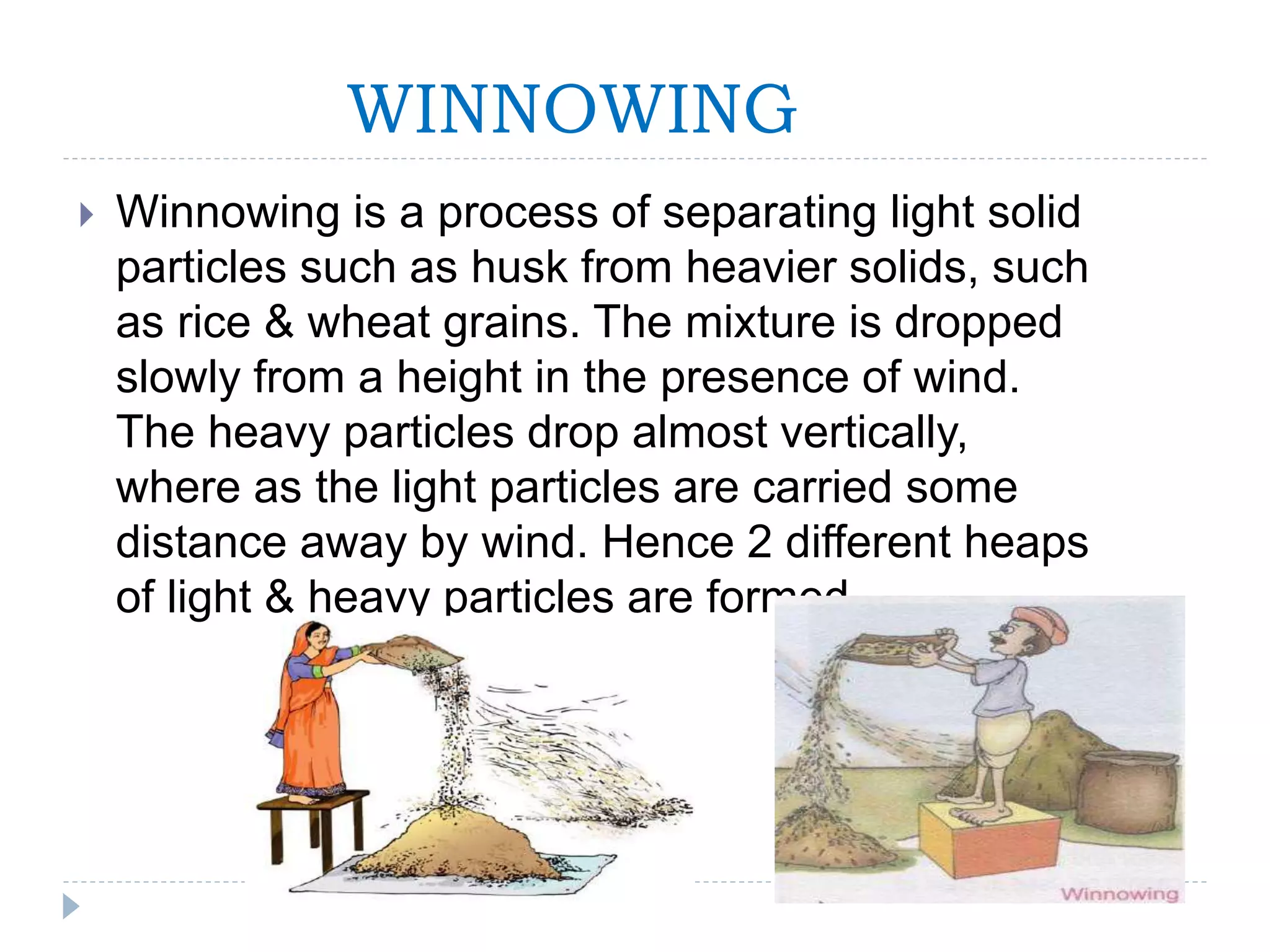
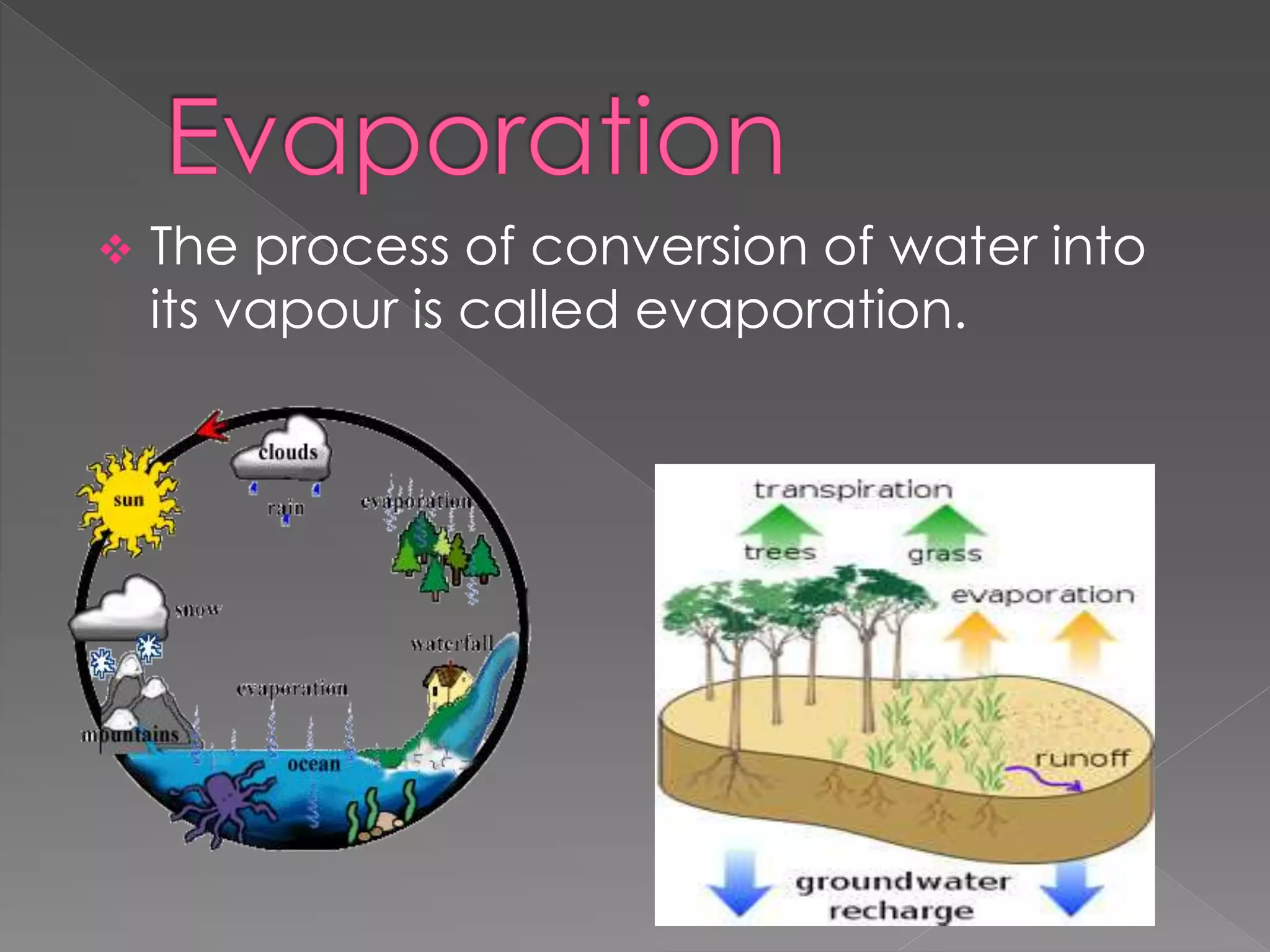
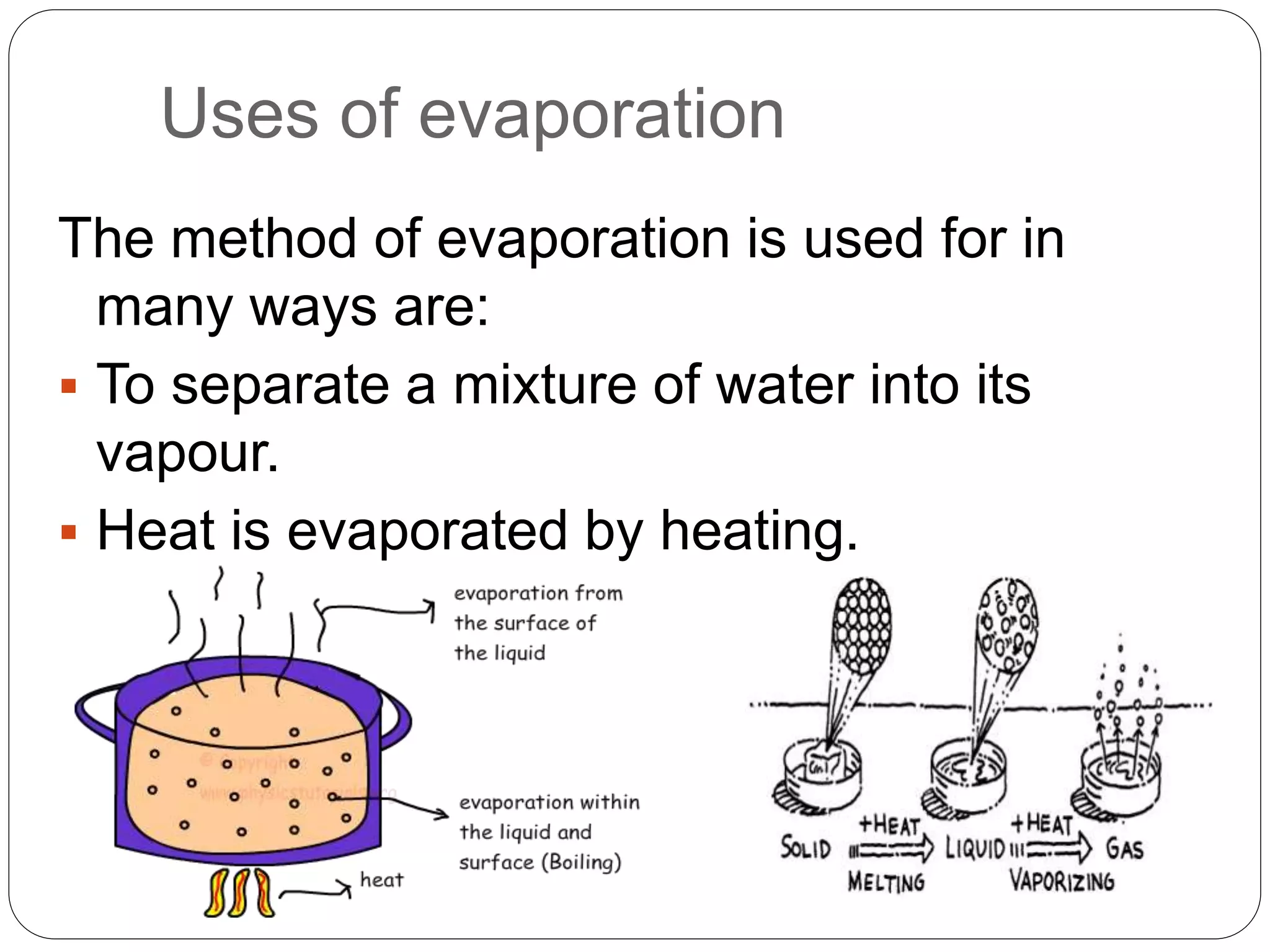
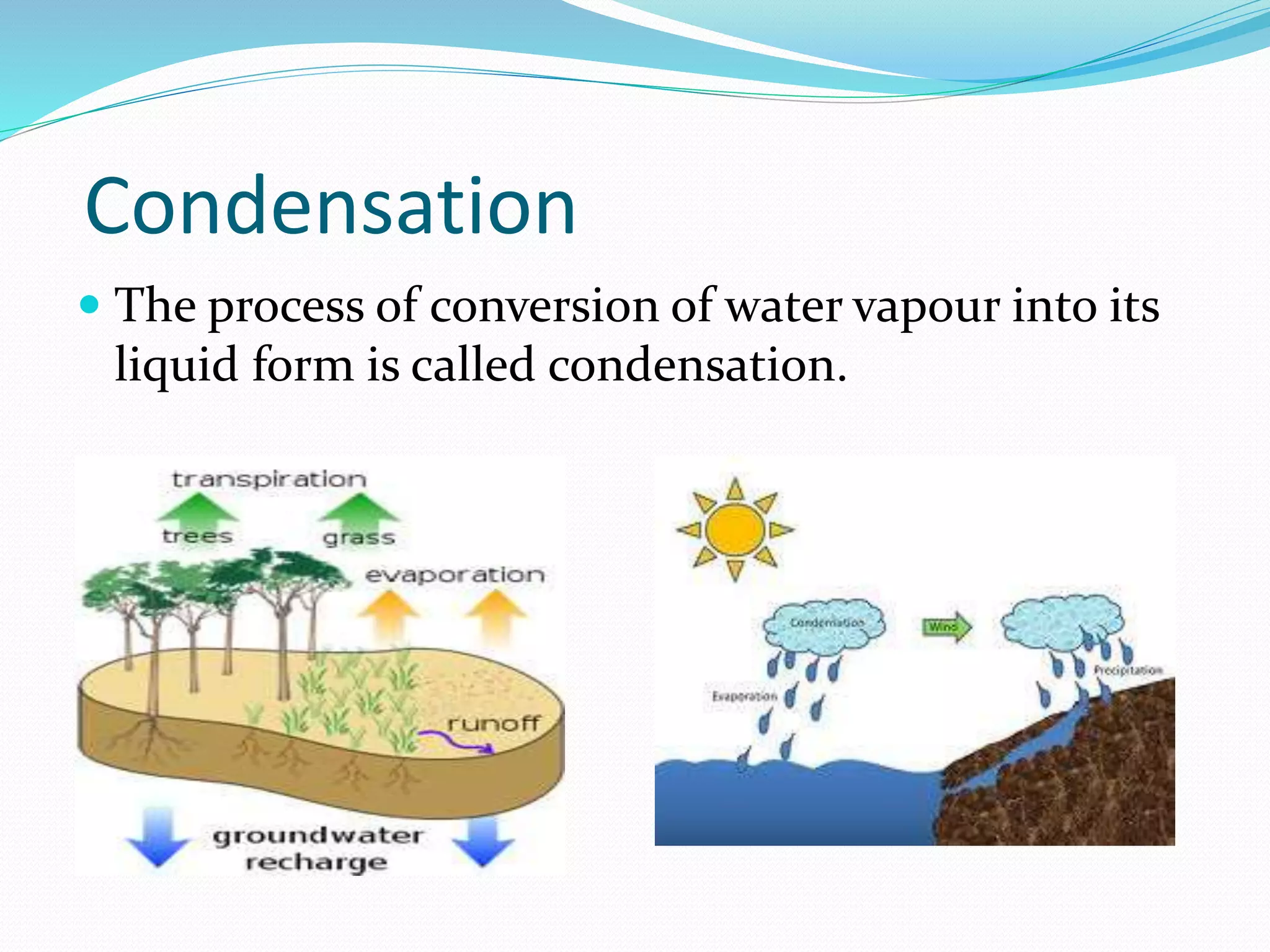
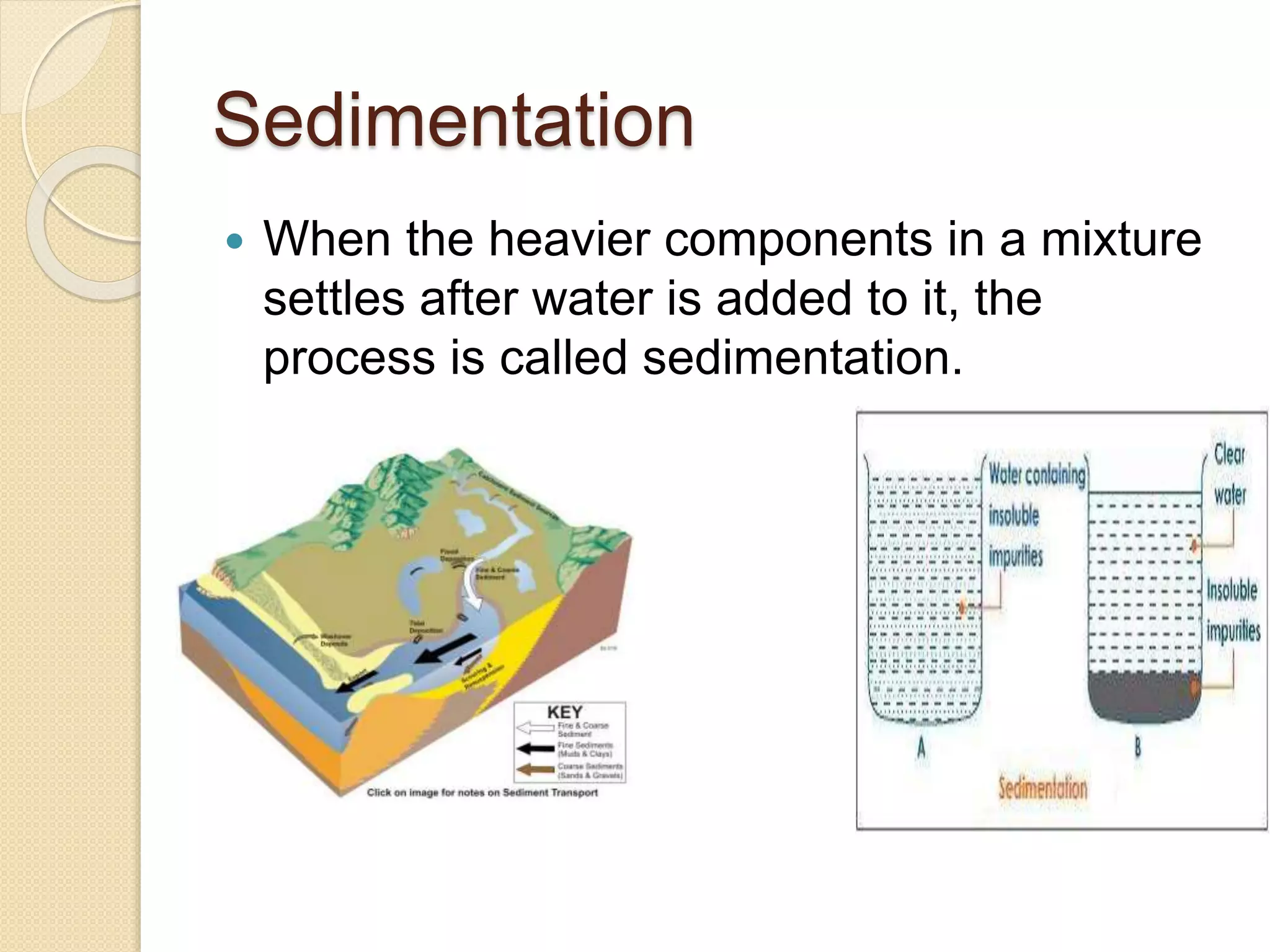
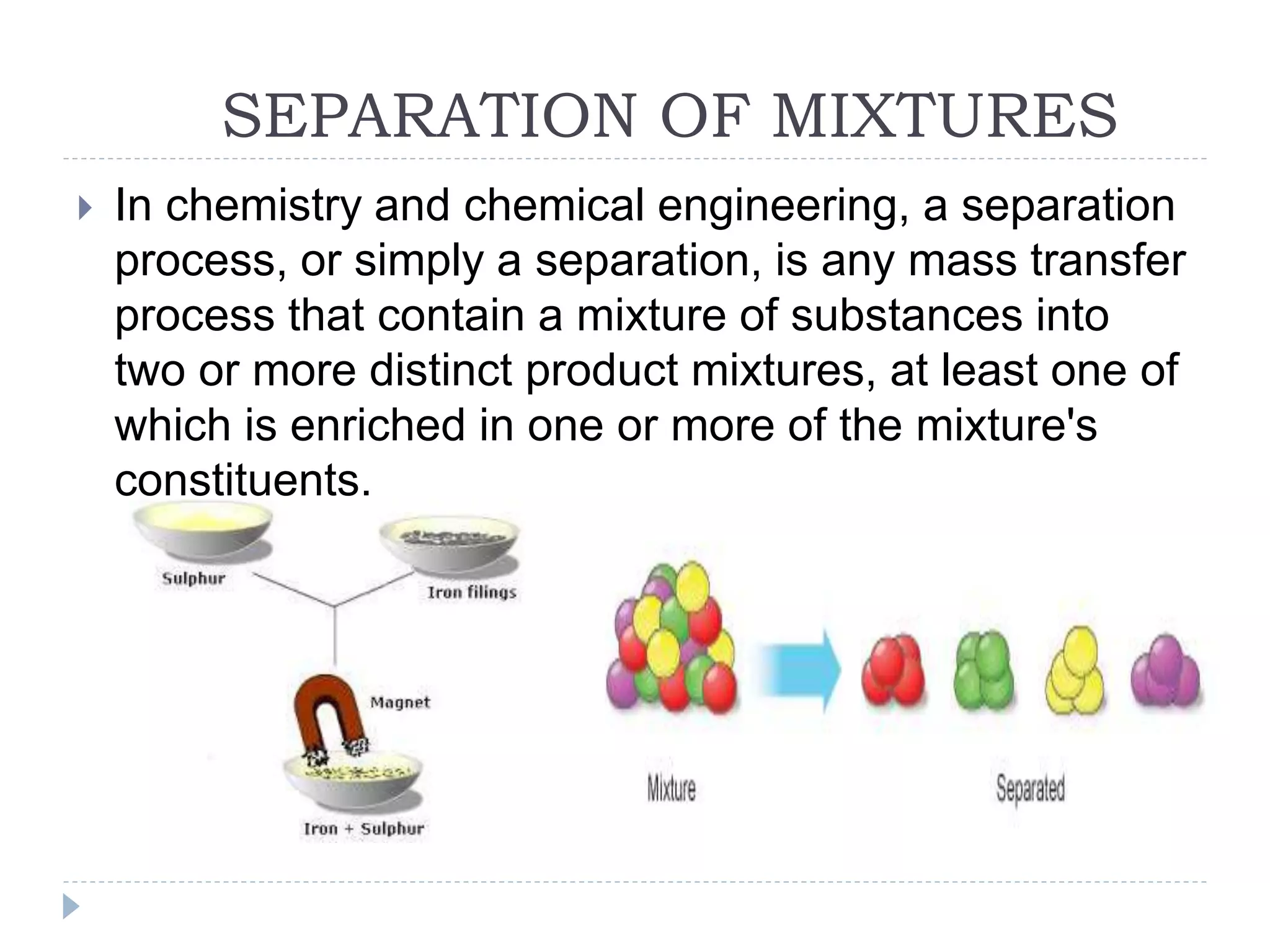
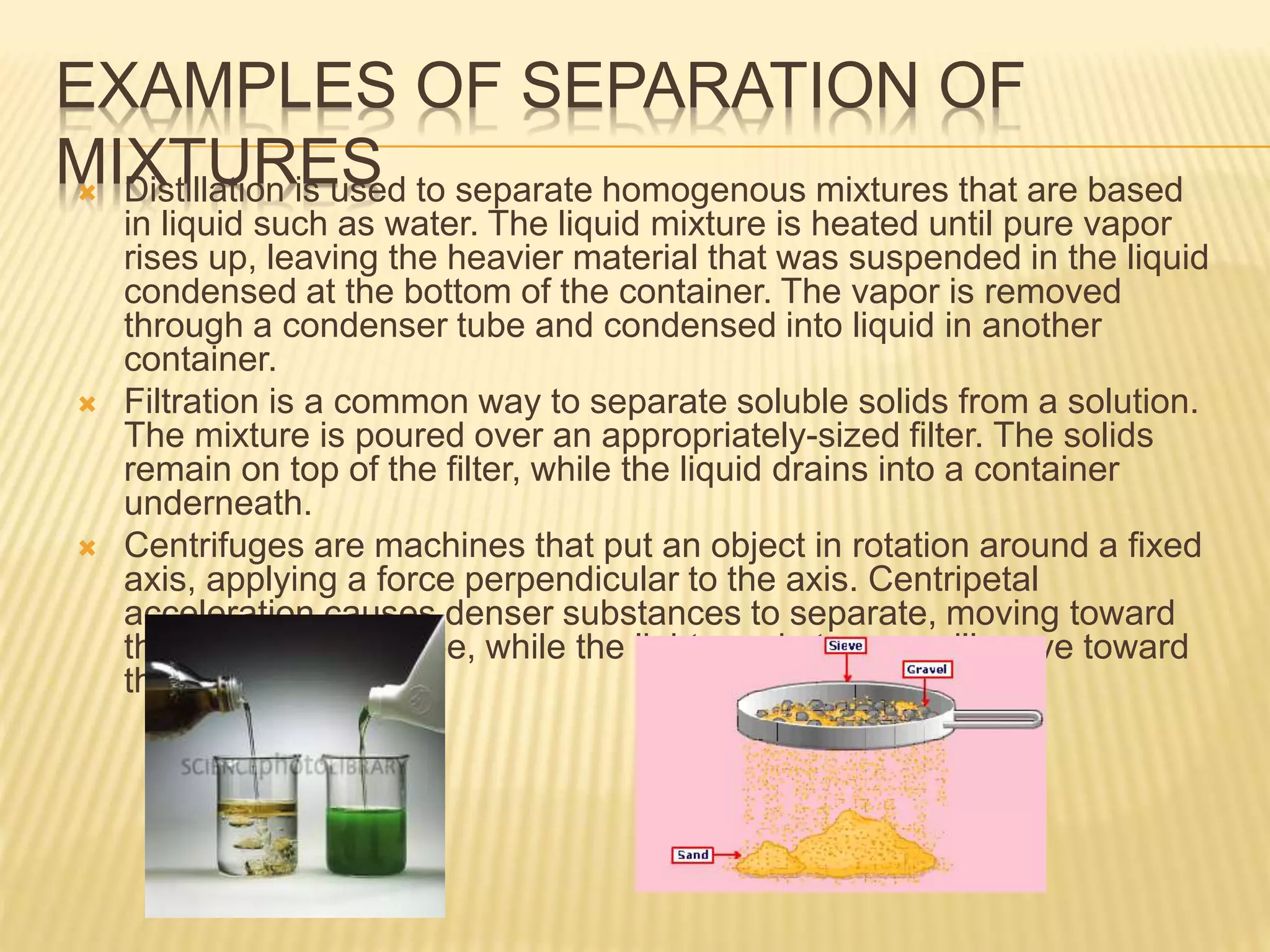
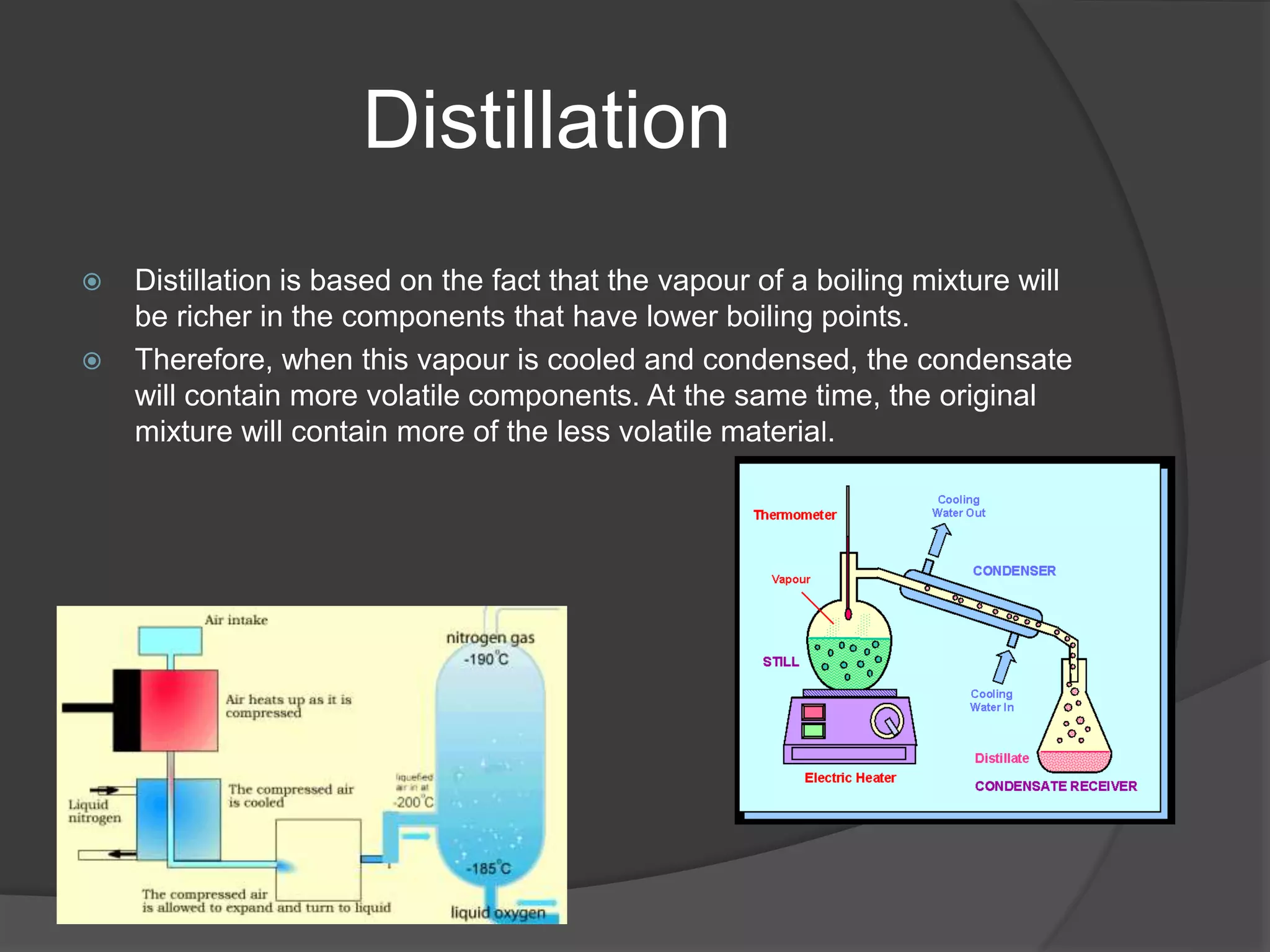
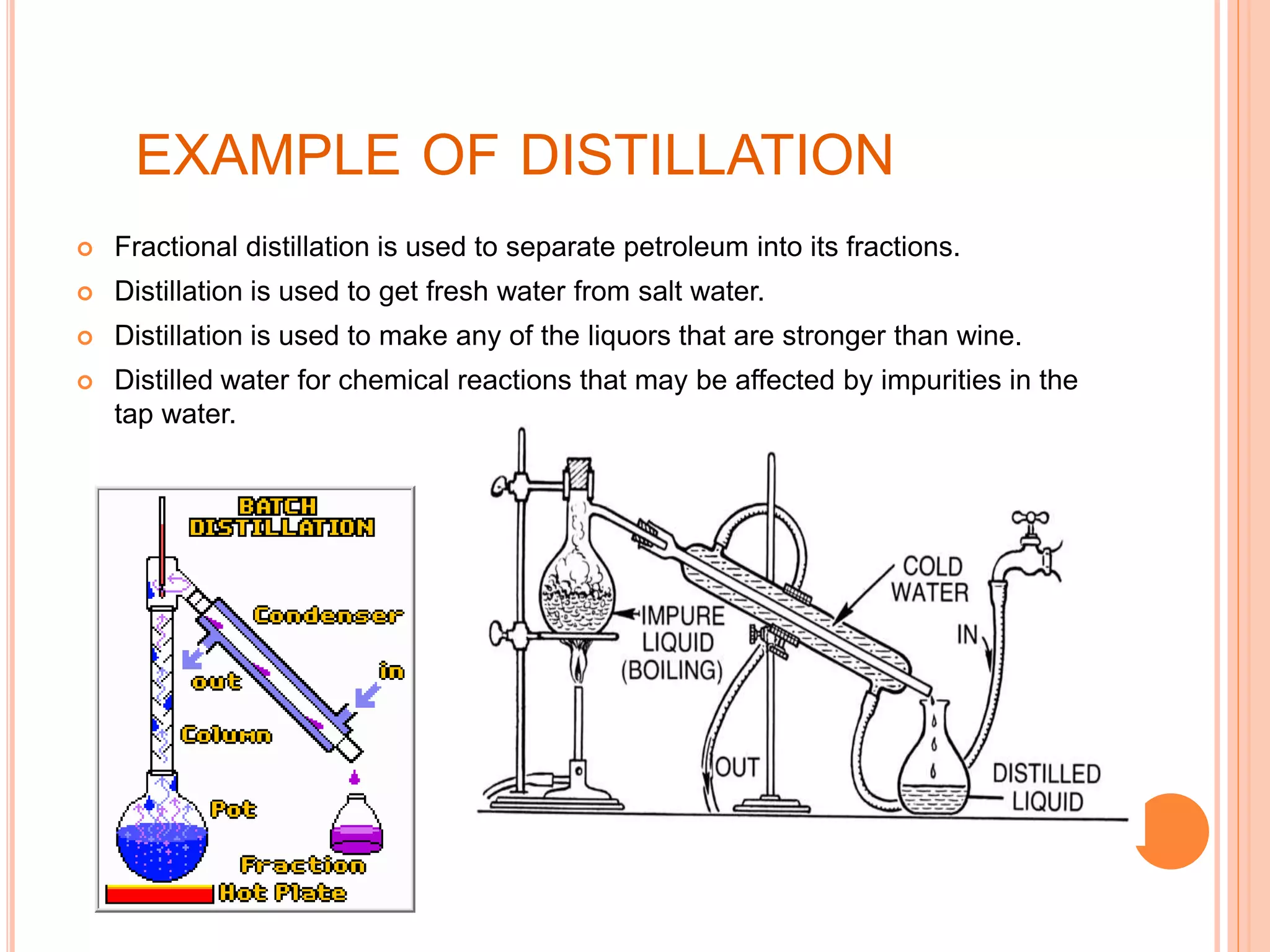
This document provides an overview of various physical separation methods used to separate components of mixtures, including handpicking, winnowing, threshing, evaporation, filtration, condensation, decantation, sedimentation, churning, straining, and water treatment. It discusses the basic process and examples of uses for each method. Distillation, centrifuges, separating funnels, and river water treatment are also summarized in more detail.













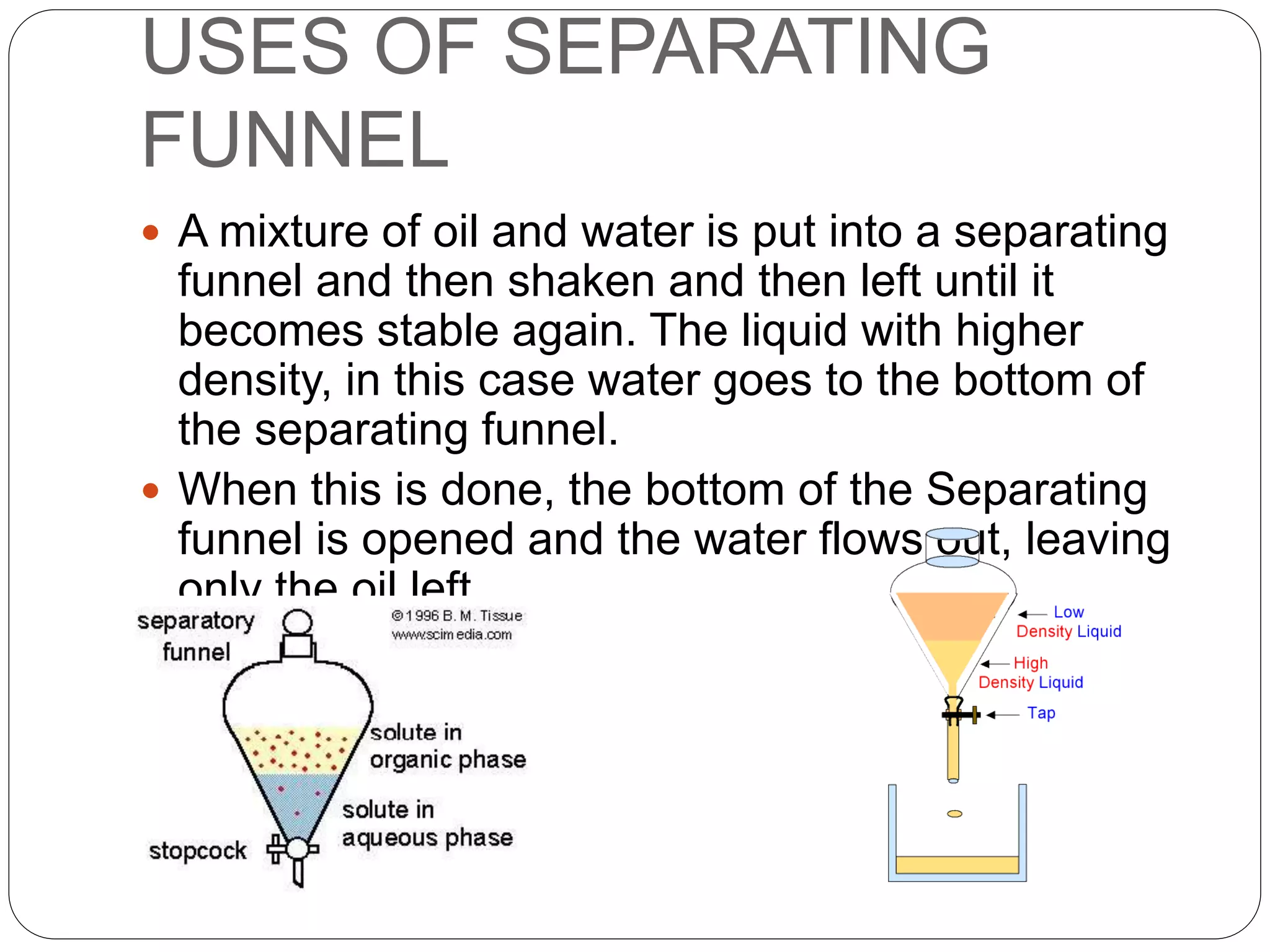
![Separating Funnel
A separatory funnel, also known as separation funnel, separating
funnel, or colloquially sep funnel, is a piece of laboratory
glassware used in liquid-liquid extractions to separate (partition)
the components of a mixture into two immiscible solvent phases
of different densities[1] Typically, one of the phases will be
aqueous, and the other a non-polar lipophilic organic solvent
such as ether, MTBE, dichloromethane, chloroform, or ethyl
acetate. All of these solvents form a clear delineation between
the two liquids](https://image.slidesharecdn.com/decbquuvqa2ovaa1uyra-signature-fe5b7916d1d4a0691d323ef5f7ad4ca390a79e290f9a81761a8335c0d04c1fd0-poli-150209230545-conversion-gate01/75/Introduction-31-2048.jpg)