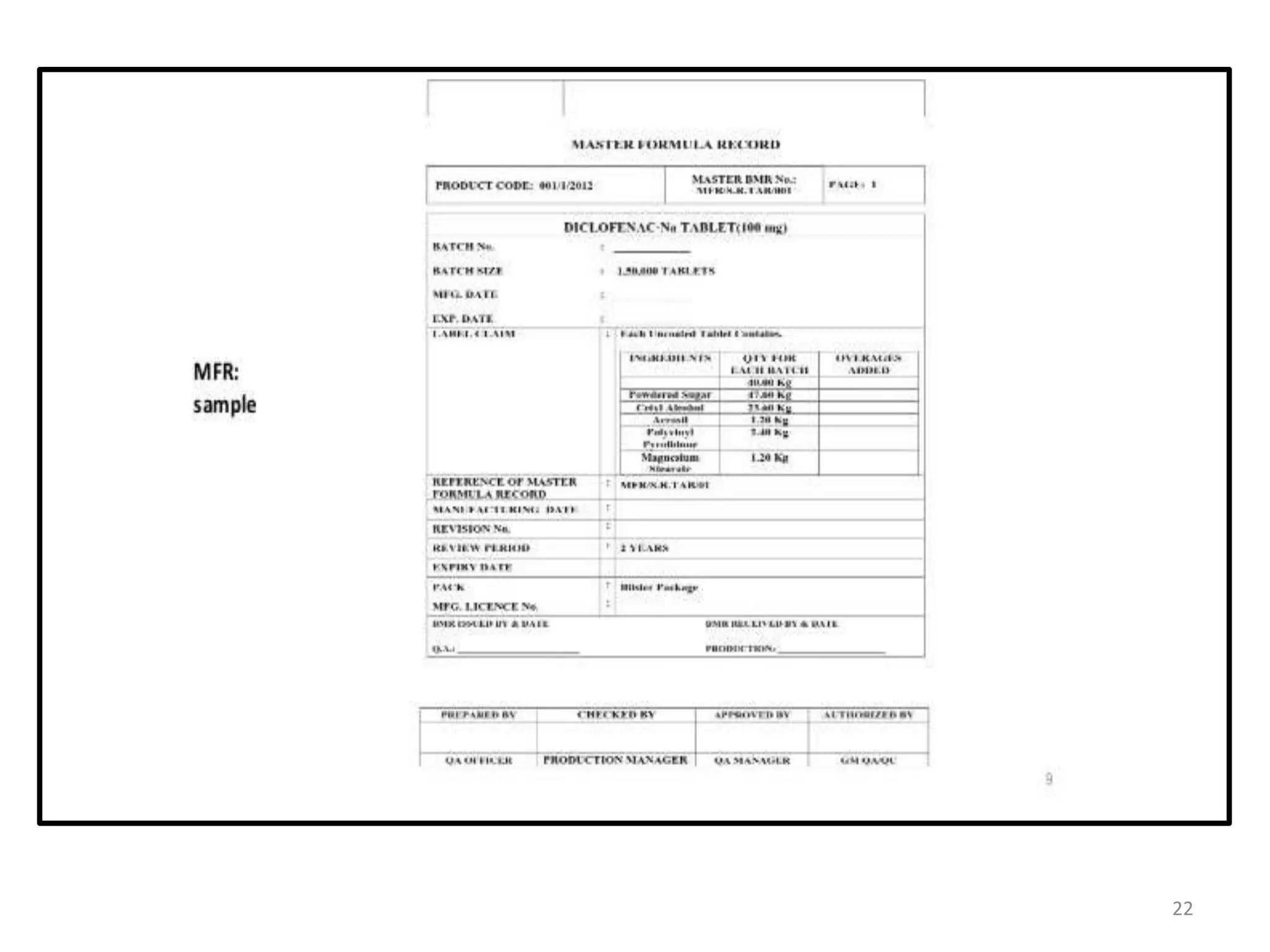
The document is a standard operating procedure (SOP) for the pharmaceutical industry, outlining its importance in ensuring quality control and consistency in processes. It details the objectives, benefits, writing style, do's and don’ts, and includes specific SOP procedures related to tablet compression and master formula records. The SOP serves as both a training tool and a guideline to comply with industry regulations, ultimately promoting safety and quality in pharmaceutical practices.