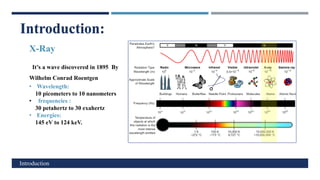
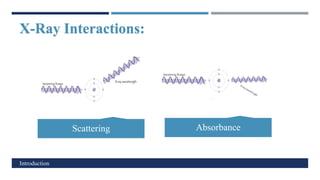
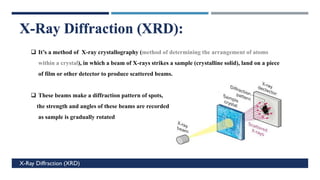
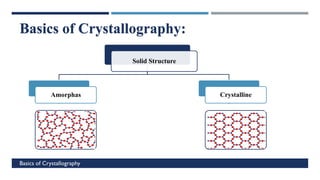
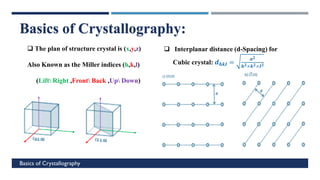
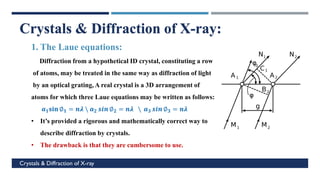
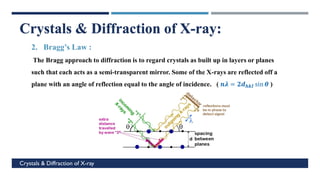
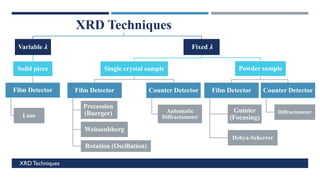
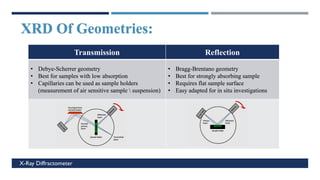
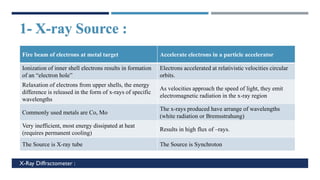
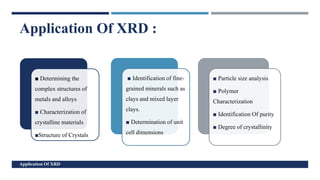
X-Ray Diffraction (XRD) is a crystallographic method for analyzing the atomic and molecular structure of crystals. XRD works by firing X-rays at a crystalline sample and measuring the angles and intensities of diffracted rays. This diffraction pattern can be used to determine the sample's crystal structure, including its unit cell dimensions and atomic positions. XRD techniques include Bragg's Law, which describes diffraction from crystal planes, and X-ray diffractometers, which contain an X-ray source, sample holder, and detector. XRD has many applications including determining crystal structures, identifying materials, and analyzing particle size and crystallinity.