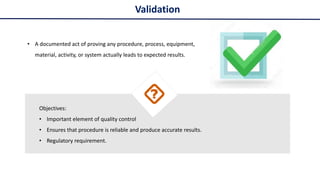
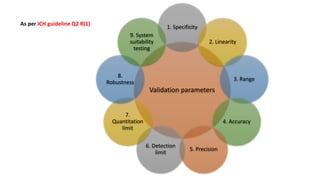
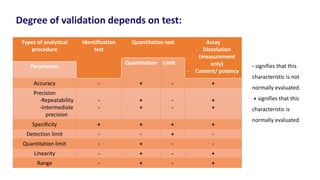
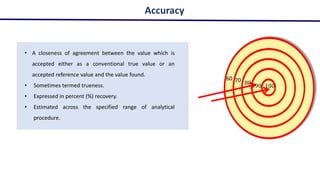
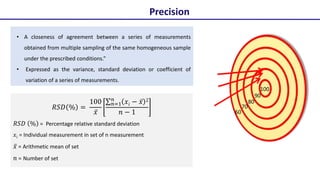
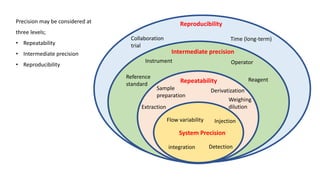
This document discusses accuracy and precision in analytical procedure validation. It defines accuracy as closeness of agreement between a measured value and a standard or known value. Accuracy is expressed as percent recovery and is determined by analyzing samples of known concentrations. Precision refers to the degree of agreement among multiple measurements under similar testing conditions and is expressed as percent relative standard deviation. Methods are considered precise if repeated measurements show similar results. The document outlines ICH guidelines for determining accuracy and precision in method validation.