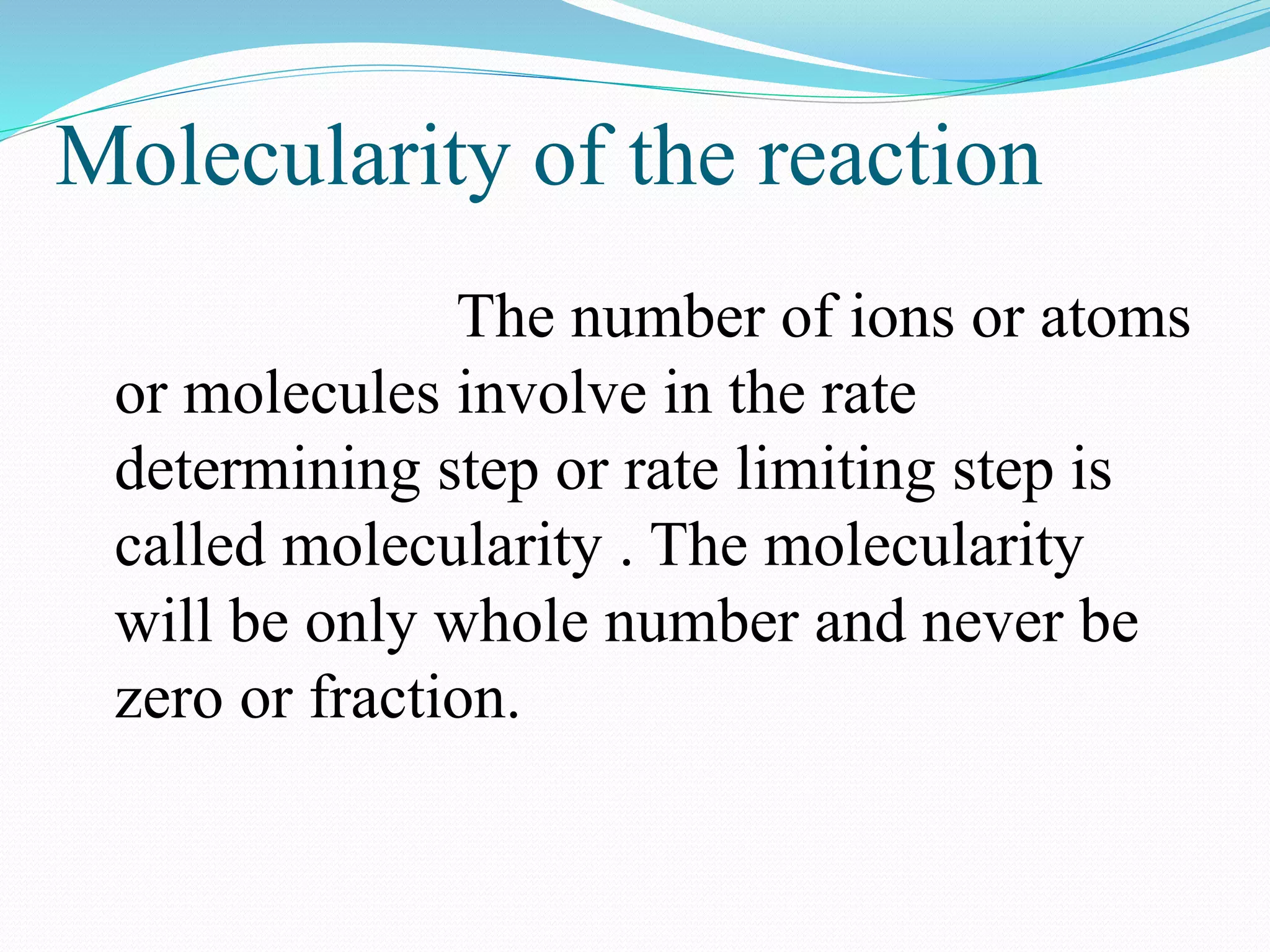
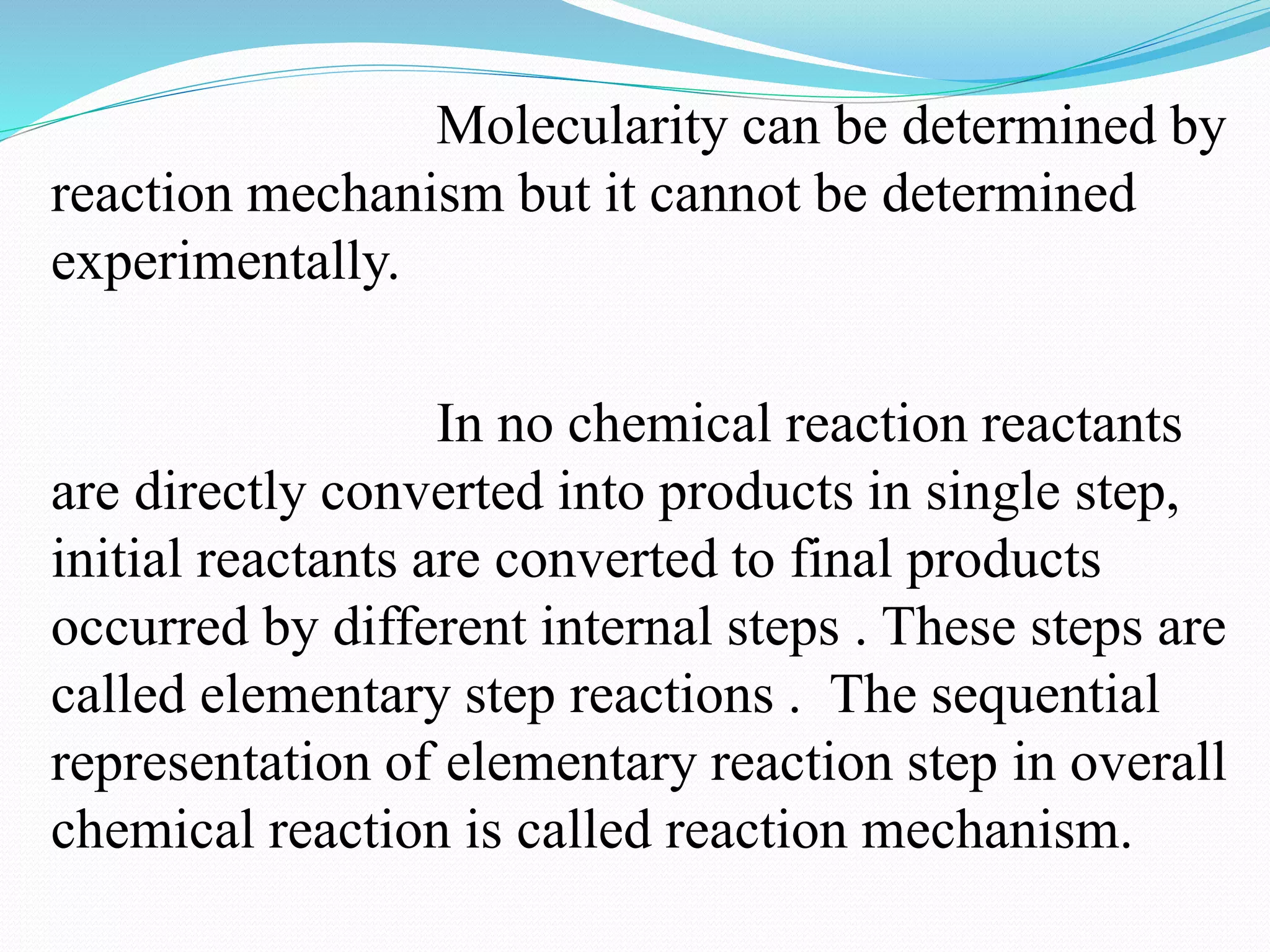
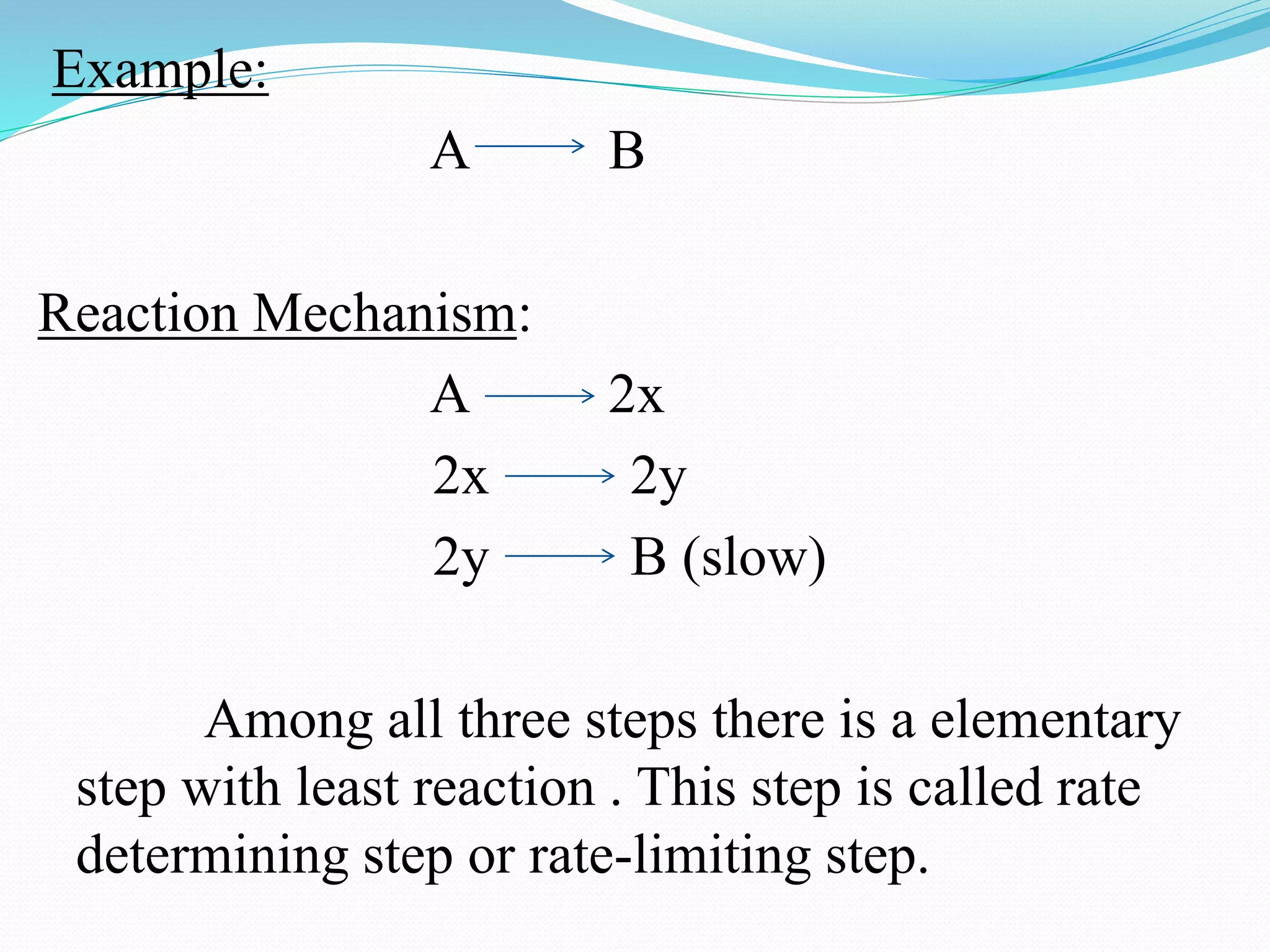
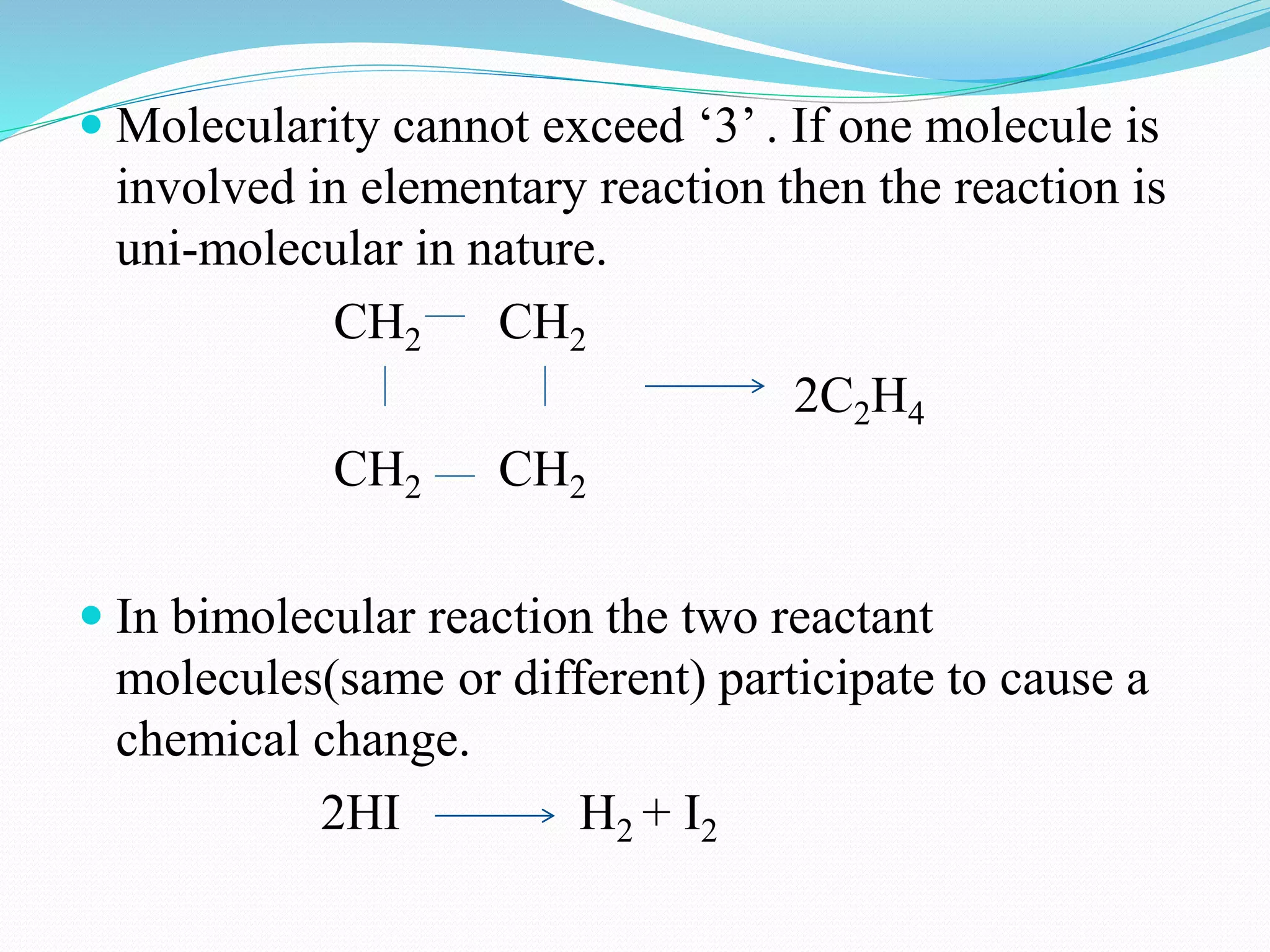
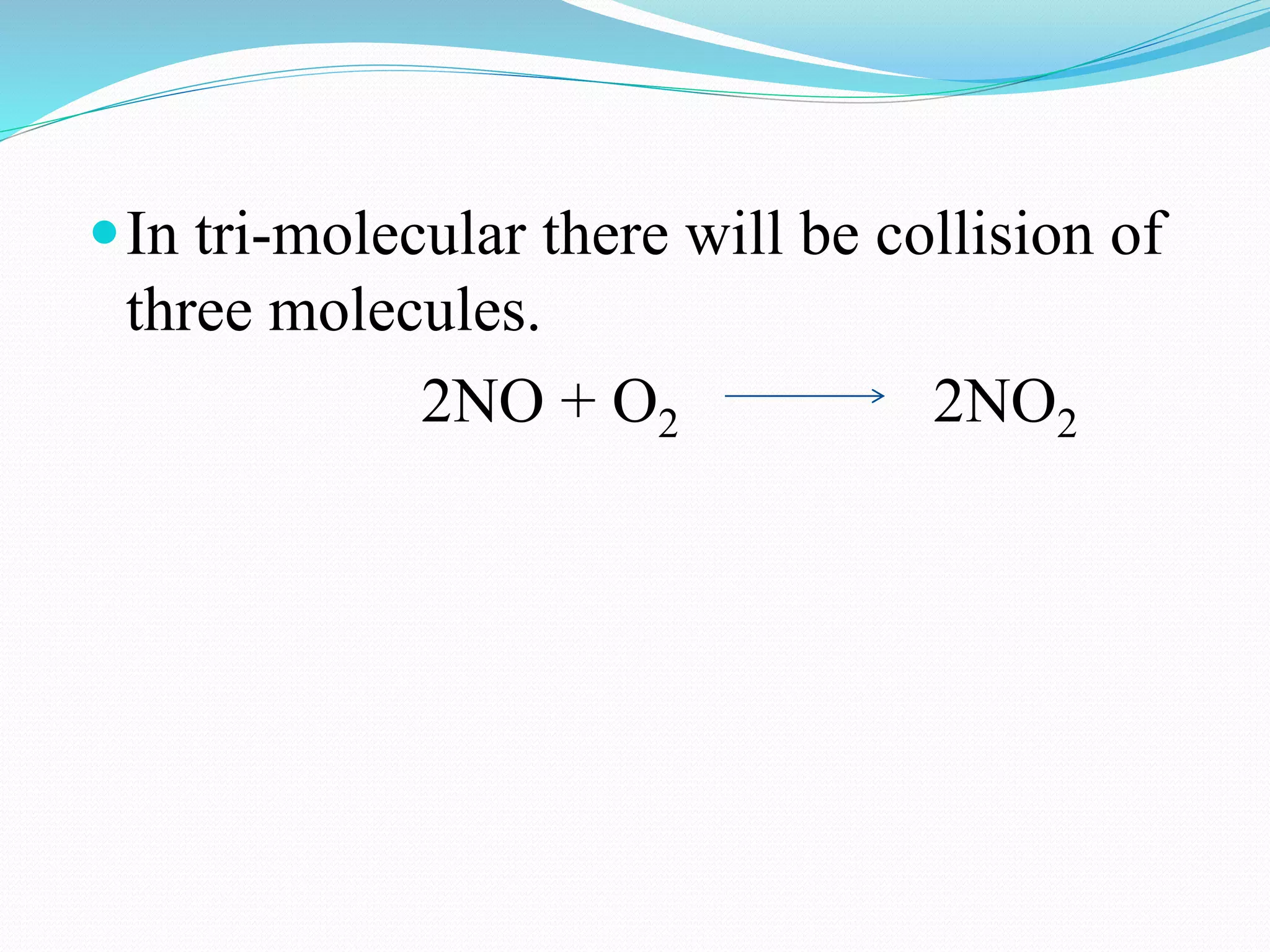
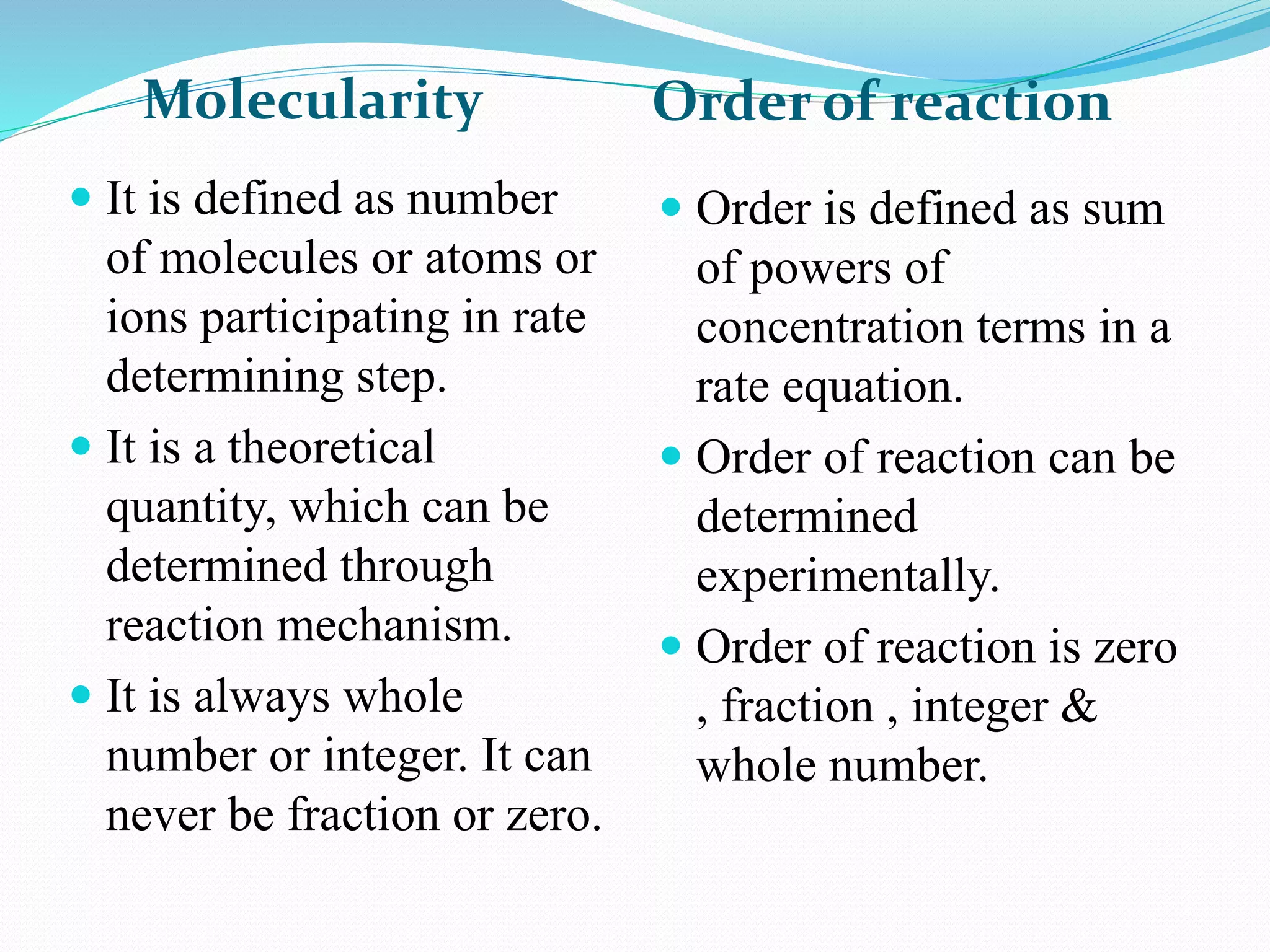
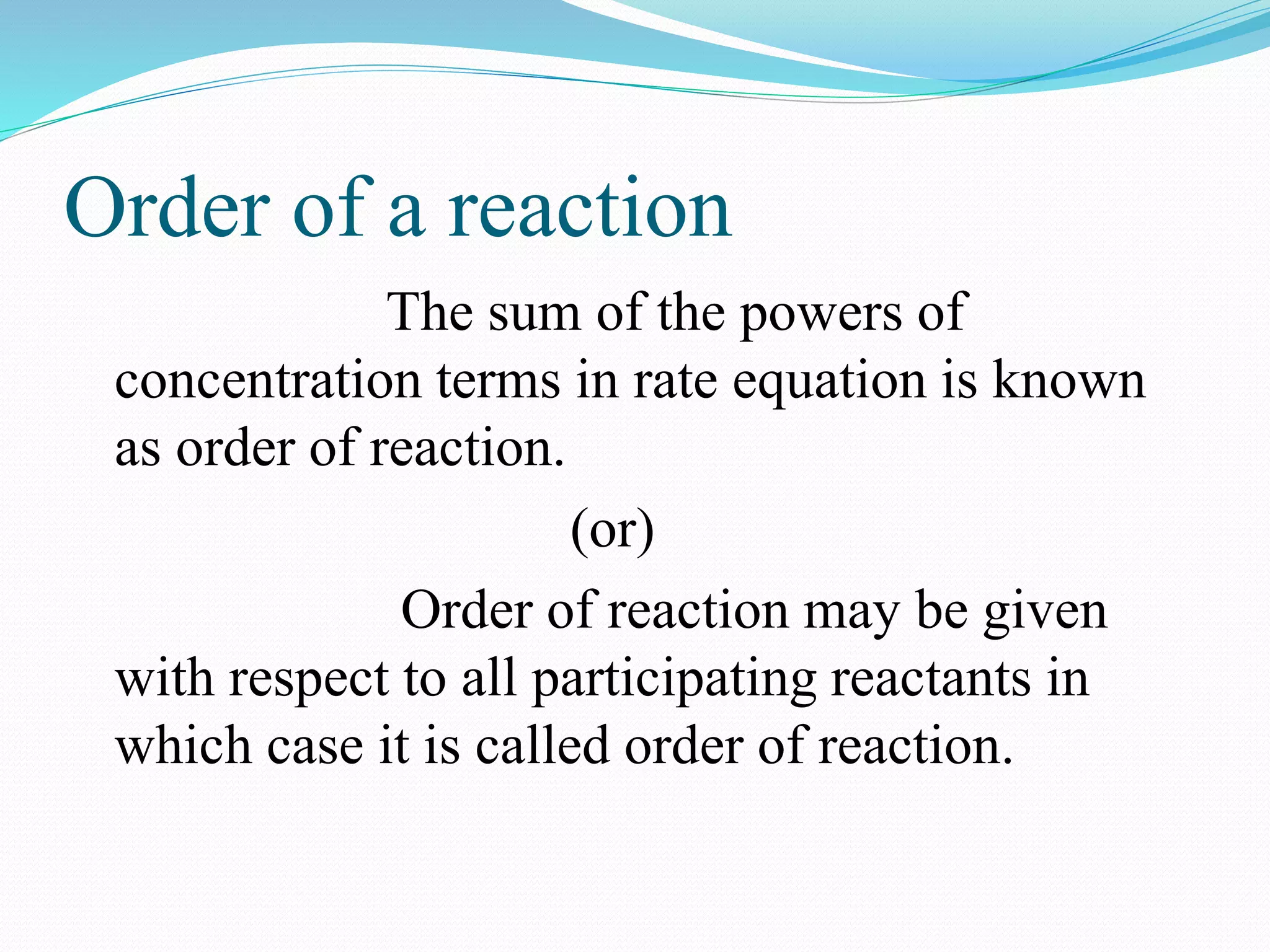
The order of a reaction refers to the sum of the powers of the concentration terms in the rate equation. It represents the number of molecules or atoms involved in the rate determining step. The document discusses zero, first, second, and third order reactions, providing examples and equations for determining the rate constant and half-life for each order. Molecularity refers to the actual number of reactant species involved in the elementary reaction step and can only be 1, 2, or 3, whereas order is a measurable property determined from the rate equation.

![Consider a reaction mA + nB product
Rate eq (R)=k[A]m[B]n
Order = m + n](https://image.slidesharecdn.com/differencebetweenorderandmolecularityofareaction2310-151029150455-lva1-app6892/75/Difference-between-order-and-molecularity-of-a-reaction-2310-3-2048.jpg)


![First order Reaction
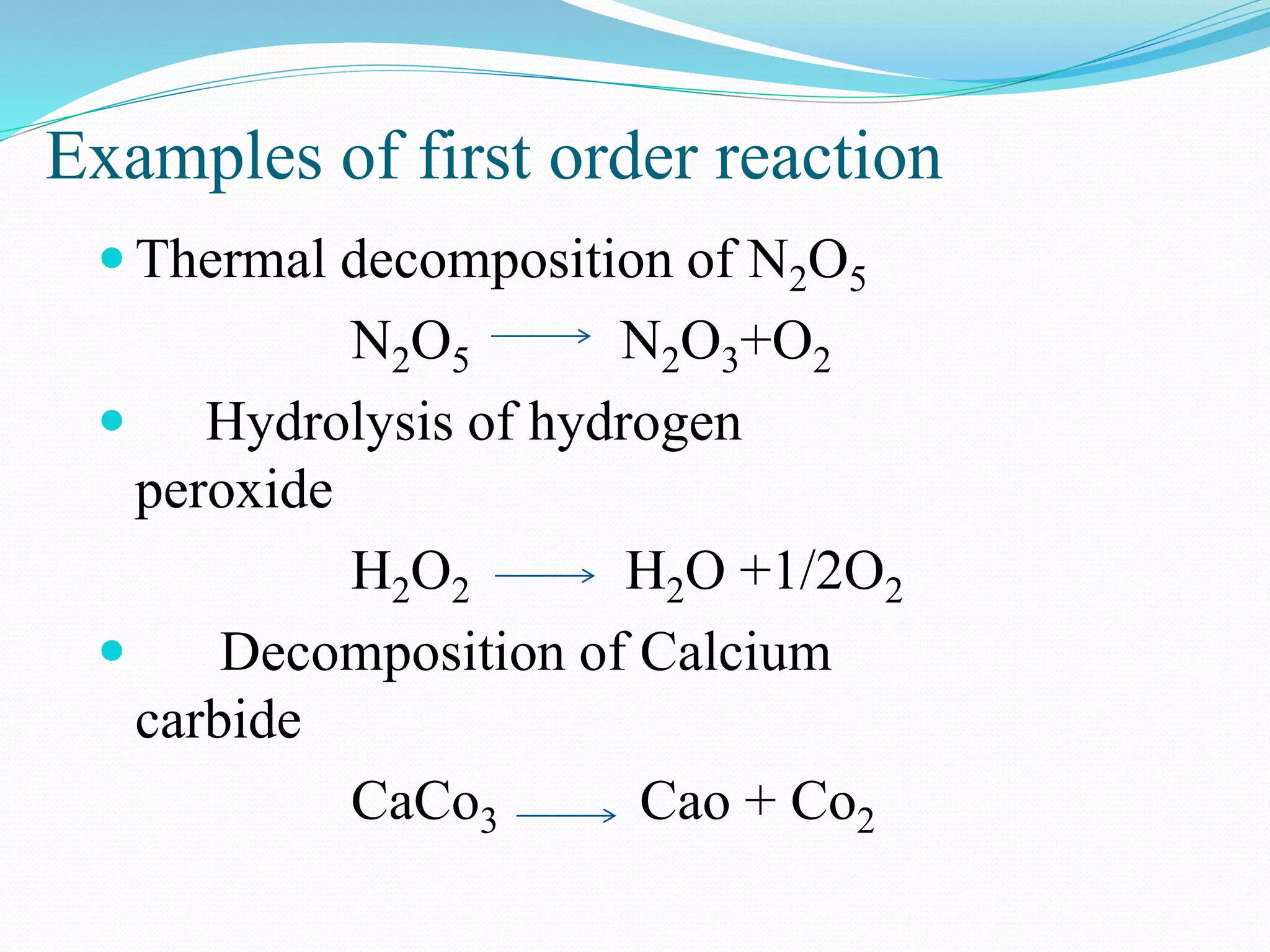
First order reaction consists of one reactant.
A product
R = k[A]1
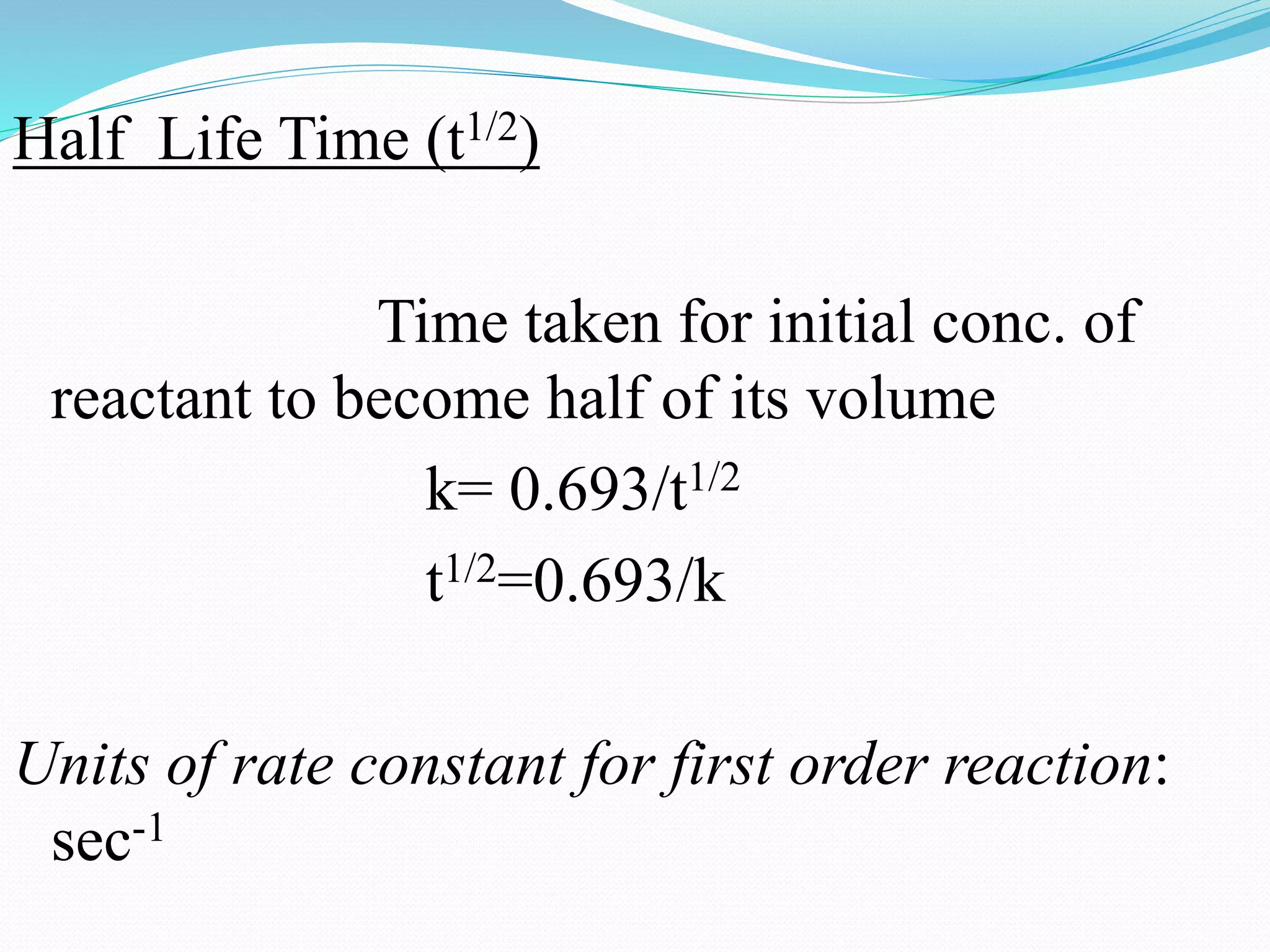
Rate constant for first order reaction:
k = 2.303 . log(a/a-x)
t
where, a=initial conc. of A in mol/lit
a-x=change in initial conc. of A in
mol/lit in time interval ’t’](https://image.slidesharecdn.com/differencebetweenorderandmolecularityofareaction2310-151029150455-lva1-app6892/75/Difference-between-order-and-molecularity-of-a-reaction-2310-6-2048.jpg)
![Second Order Of Reaction
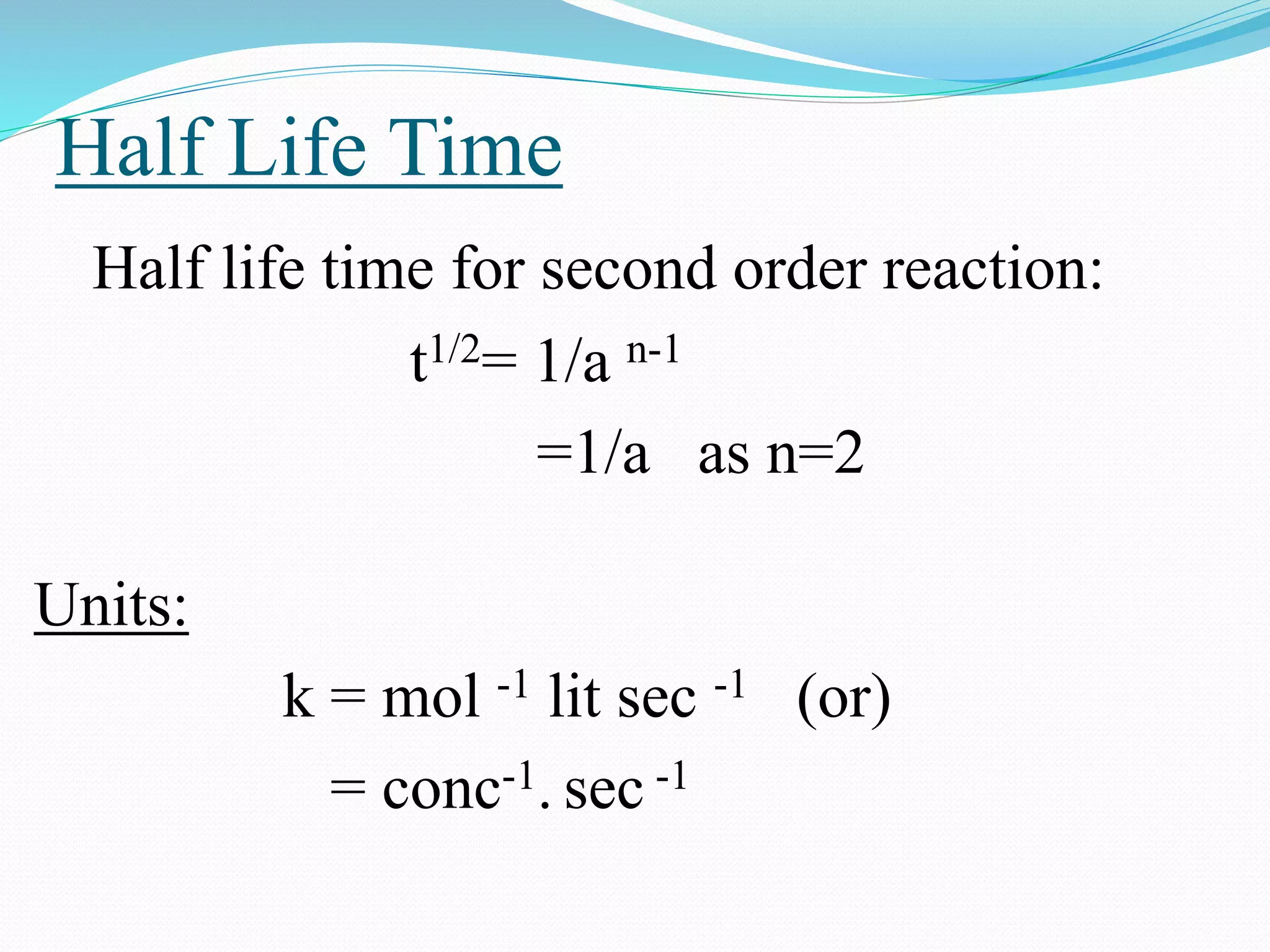
Second order reaction consists
of two reactants.
2A products
A+B products
Rate = k[A]2 ; n=2
Rate = k[A] [B] ; n=1+1=2](https://image.slidesharecdn.com/differencebetweenorderandmolecularityofareaction2310-151029150455-lva1-app6892/75/Difference-between-order-and-molecularity-of-a-reaction-2310-9-2048.jpg)
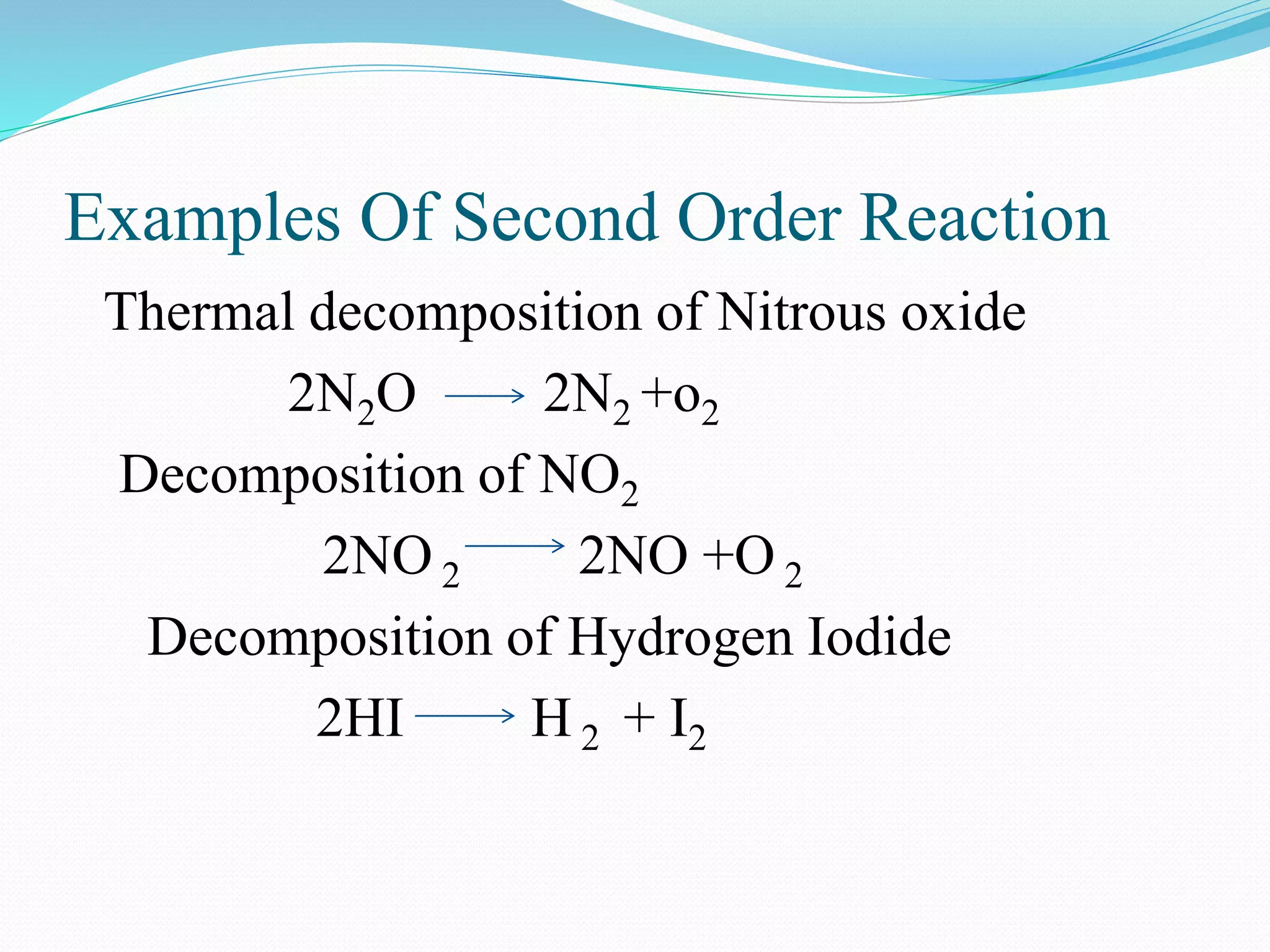
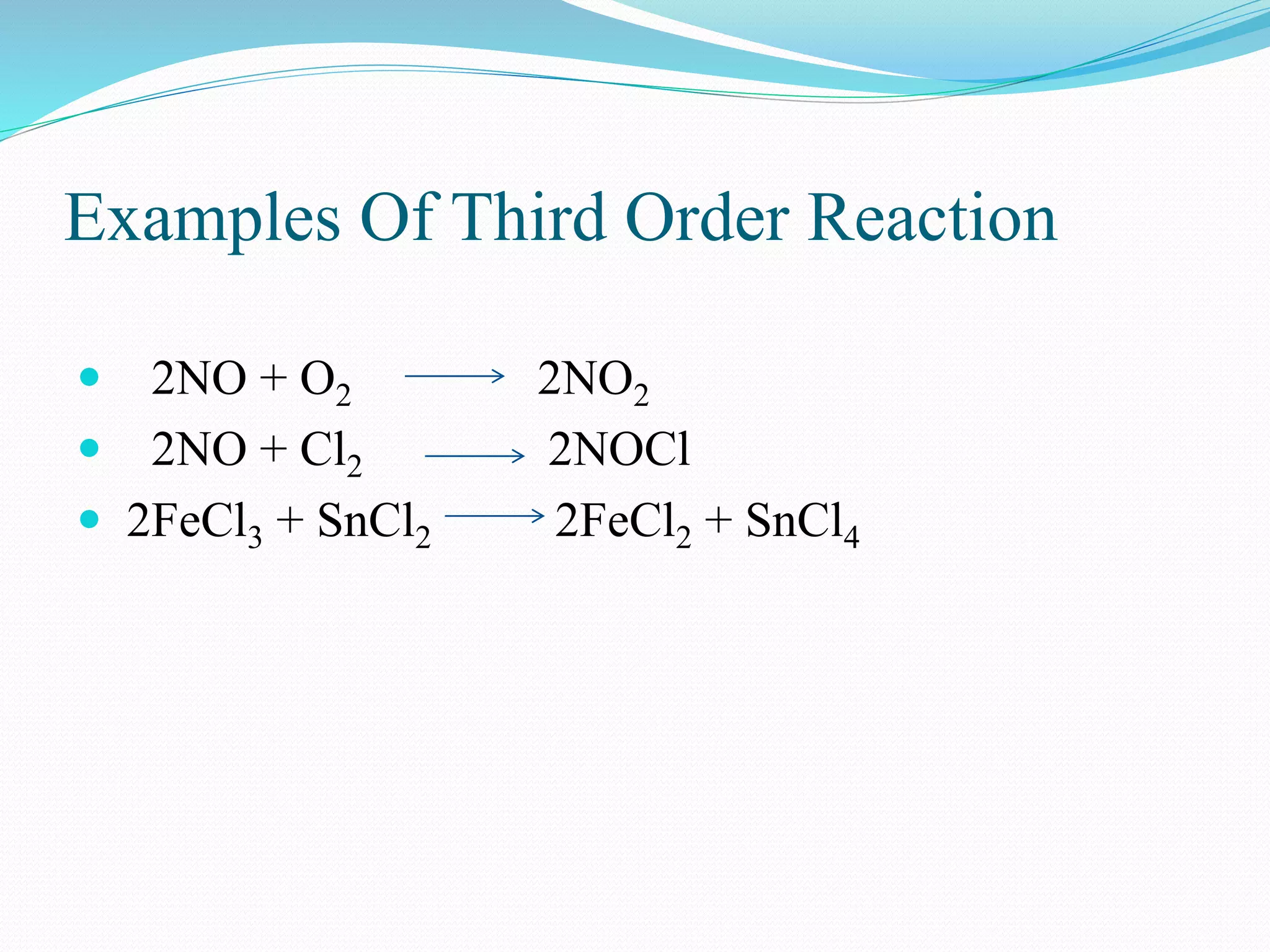
![Third Order Reaction
Third order reaction includes three
reactants
3A Products
r = k [A] 3
A+B+C Products
r = k [A] [B] [C]
2A+B Products
r = k [A]2 [B]](https://image.slidesharecdn.com/differencebetweenorderandmolecularityofareaction2310-151029150455-lva1-app6892/75/Difference-between-order-and-molecularity-of-a-reaction-2310-12-2048.jpg)
![Half Life Time
Half life time for third order reaction
t ½ = 1/a n-1
= 1/a 3-1 = 1/ a2
[as n=3]
Units:
k = mol -2 lit 2 sec -1
= conc. -2 sec -1](https://image.slidesharecdn.com/differencebetweenorderandmolecularityofareaction2310-151029150455-lva1-app6892/75/Difference-between-order-and-molecularity-of-a-reaction-2310-13-2048.jpg)