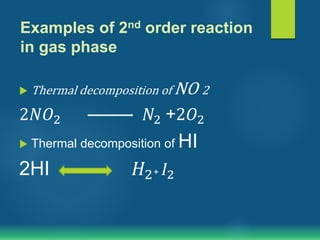
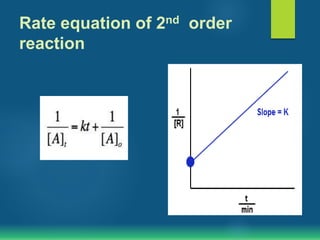
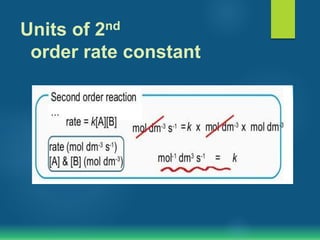
The document discusses second order reactions, explaining that the reaction rate depends on the concentration of two reactants and provides rate equations for different scenarios. It includes examples of second order reactions both in gas and solution phases, as well as their relevance in biological processes. The half-life of second order reactions is also detailed, indicating its dependence on initial concentration.



![2nd order reaction with different initial
concentration of reactant
Initial conc. of A and B are a and b and after time t
(a-x) and (b-x) are left , so
𝑑𝑥
𝑑𝑡
= 𝑘 𝐴 𝐵
𝑑𝑥
𝑑𝑡
= 𝑘 𝑎 − 𝑥 𝑏 − 𝑥
Then ,after integration
1
(𝑎−𝑏)
[ln
𝑏.(𝑎−𝑥)
𝑎.(𝑏−𝑥)
]= kt](https://image.slidesharecdn.com/2ndorderreaction-200623053858/85/2nd-order-reaction-6-320.jpg)
![2nd order reaction with equal
concentration of reactant
Initial conc. of A and B are mole/dm-3 and after
time t (a-x) are left for both
𝑑𝑥
𝑑𝑡
= k [A][B]
𝑑𝑥
𝑑𝑡
= 𝑘 𝑎 − 𝑥 𝑎 − 𝑥
= k(𝑎 − 𝑥)2
Then, after integration
𝑥
𝑎(𝑎−𝑥)
= kt](https://image.slidesharecdn.com/2ndorderreaction-200623053858/85/2nd-order-reaction-7-320.jpg)
![Half life and second order
reaction
If conc. Are same, then
kt =
𝑥
𝑎(𝑎−𝑥)
t =
1
𝑘
.
when , x =
𝑎
2
𝑡ℎ𝑒𝑛, t=
𝑡
2
So, 𝑡2
1
=
1
𝑘
.
𝑎
2
𝑎(𝑎−
𝑎
2
)
[t1
2]2 =
1
𝑘𝑎
𝑥
𝑎(𝑎 − 𝑥)
The half life depends
on initial concentration
‘a’
If k and k’ are same
then order is second](https://image.slidesharecdn.com/2ndorderreaction-200623053858/85/2nd-order-reaction-8-320.jpg)