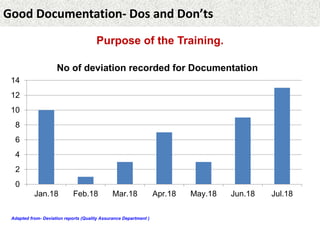
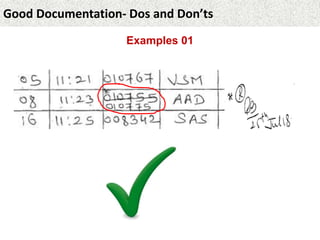
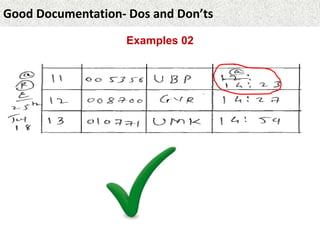
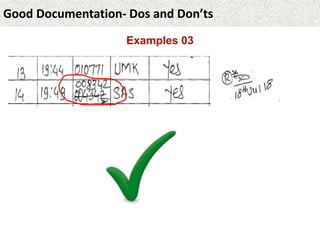
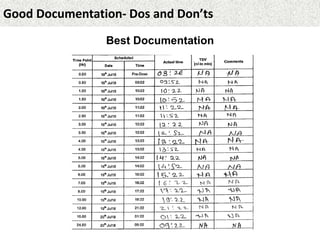
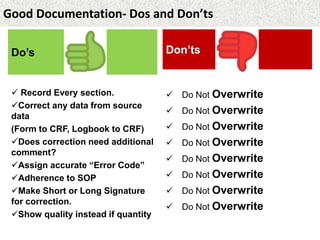
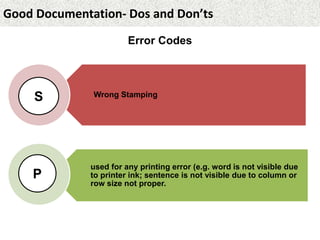
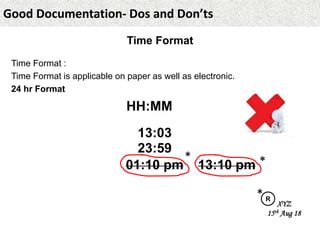
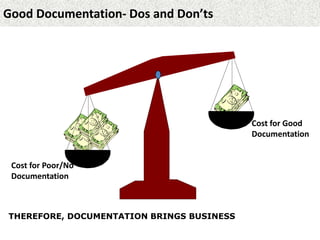
This document provides guidance on good documentation practices. It emphasizes that documentation should be attributable, legible, contemporaneous, original, and accurate. Key points include:
- Documentation is important for regulatory compliance and represents the quality of an organization's work.
- Documentation must clearly show who performed and observed activities and when.
- Original documents should be distinguishable from copies and have clear, concise information.
- Data entries must be made at the time of the activity and any corrections clearly shown.