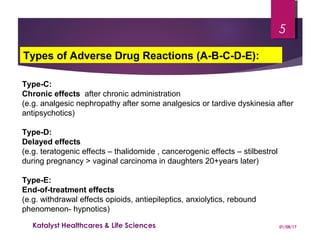
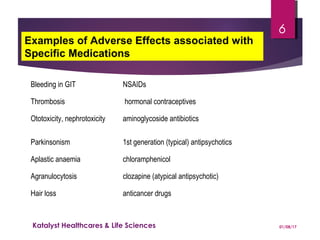
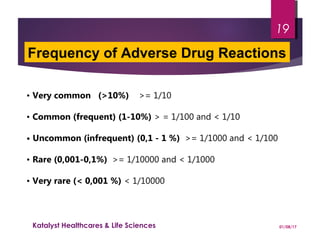
The document discusses adverse drug reactions (ADRs) and the importance of pharmacovigilance in monitoring the safety of medications. It categorizes ADRs into different types based on their nature and provides examples of specific drug-related adverse effects. Additionally, it outlines the processes for reporting and evaluating ADRs, emphasizing the need for healthcare professionals to report suspected ADRs to ensure patient safety.