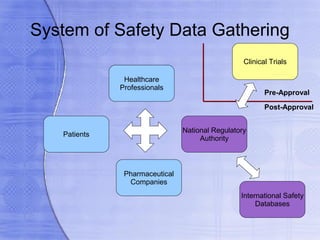
This document discusses pharmacovigilance and drug safety. It provides examples of drugs that have been withdrawn from the market in the US since 2000 due to safety issues like hepatotoxicity and cardiovascular toxicity. It also discusses key terms related to adverse drug reactions and how causality is assessed. Pharmacovigilance aims to identify safety issues with medicines through post-marketing surveillance.















![Causality Assessment
NA RANJO's ALGORITHM
question Yes No Don't know
Are there previous conclusion reports on this reaction? +1 0 0
Did the adverse event appear after the suspect drug was administered? +2 -1 0
Did the AR improve when the drug was discontinued or a specific
+1 0 0
antagonist was administered?
Did the AR reappear when drug was readministered? +2 -1 0
Are there alternate causes [other than the drug] that could solely have
-1 +2 0
caused the reaction?
Did the reaction reappear when a placebo was given? -1 +1 0
Was the drug detected in the blood [or other fluids] in a concentration
+1 0 0
know n to be toxic?
Was the reaction more severe when the dose was increased, or less
+1 0 0
severe when the dose was decreased?
Did the patient have a similar reaction to the same or similar drugs in any
+1 0 0
previous exposure?
Was the adverse event confirmed by objective evidence? +1 0 0](https://image.slidesharecdn.com/pharmacovigilancessh-121118060325-phpapp01/85/Pharmacovigilance-17-320.jpg)