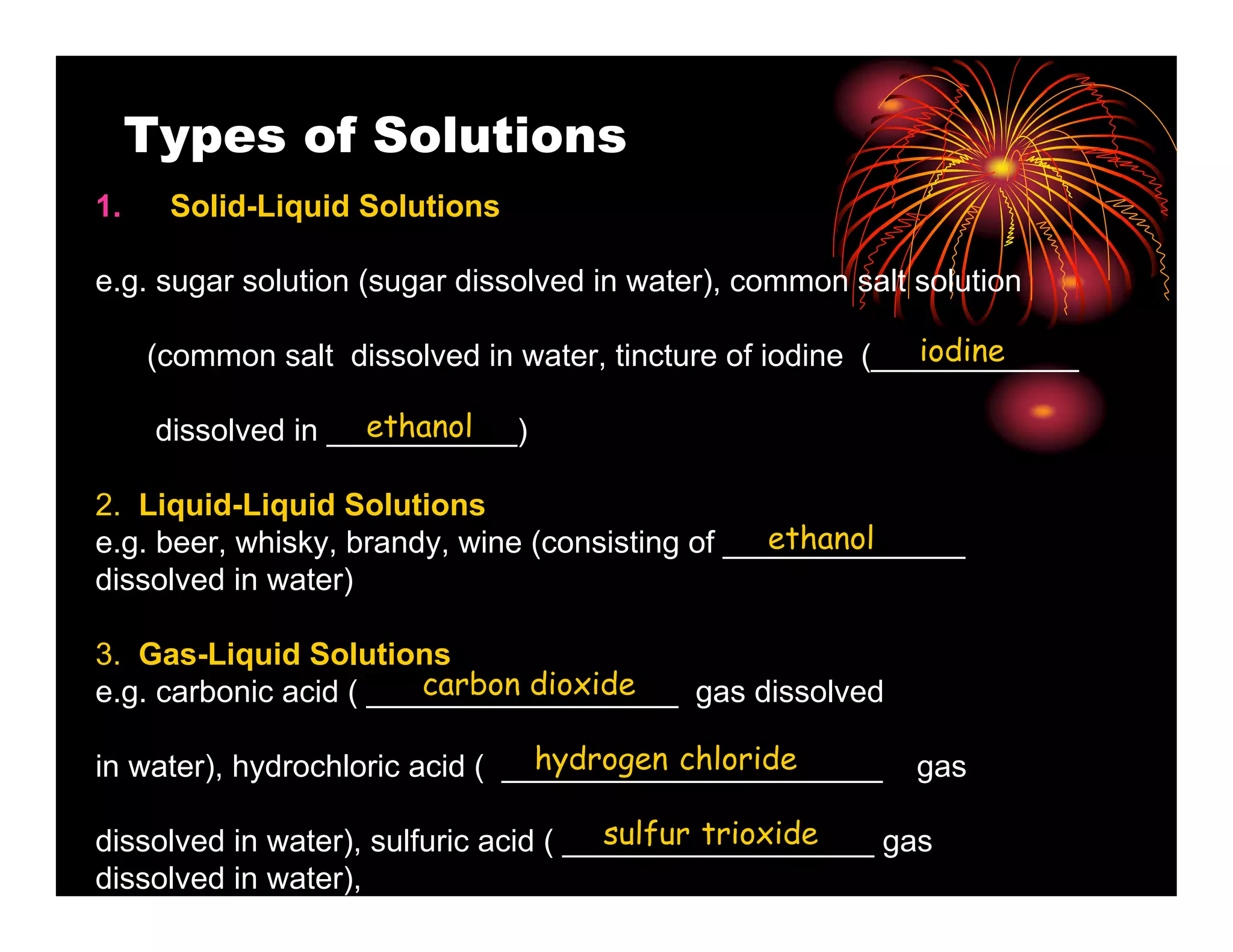
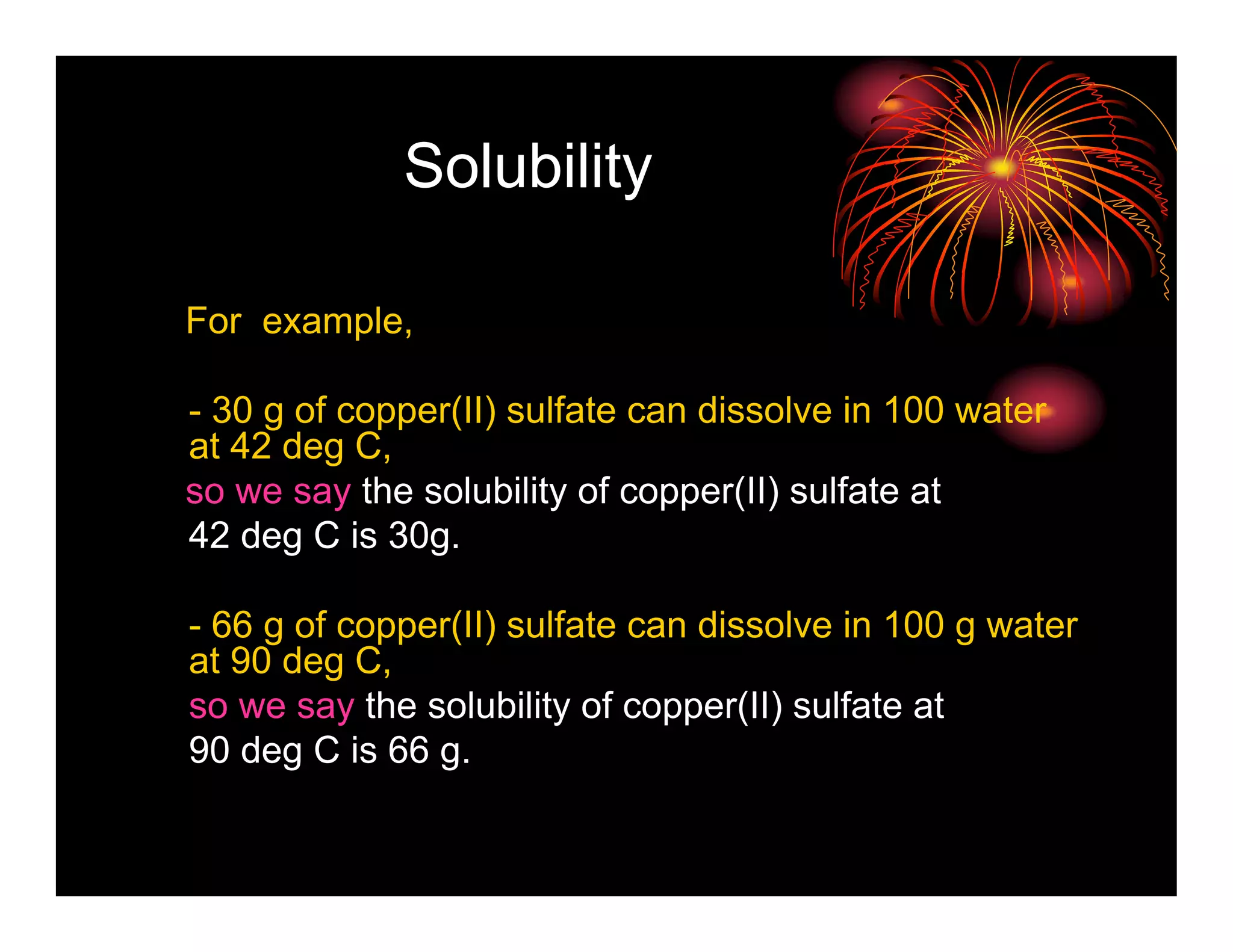
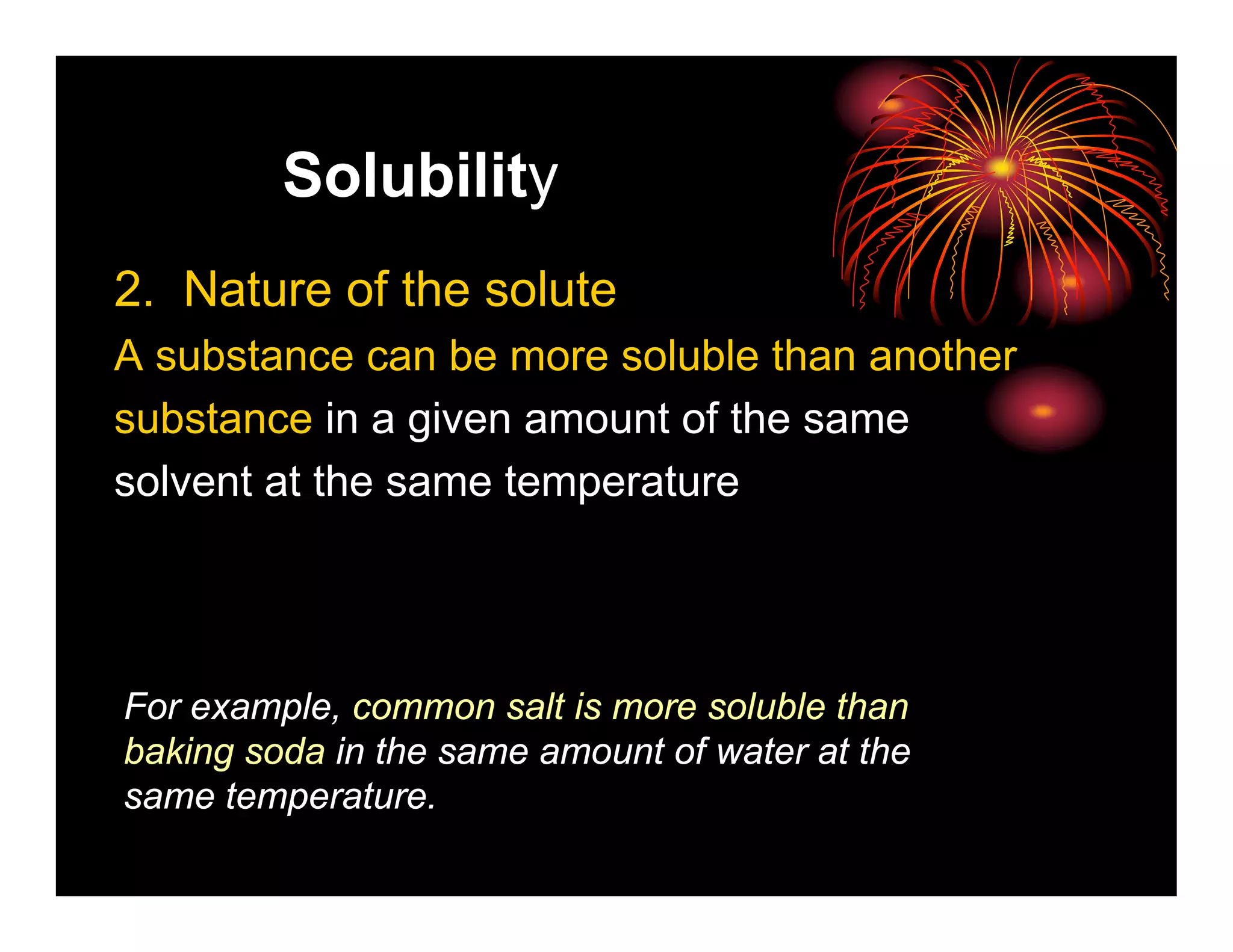
This document provides information about solutions and solubility. It defines key terms like solute, solvent, saturated solution, and solubility. It explains that a solution is a homogeneous mixture of substances where one substance dissolves in another. Water is called the universal solvent because it can dissolve more substances than any other solvent. Not all substances are soluble and solubility depends on temperature, the nature of the solute and solvent, and other factors. The document also discusses different types of solutions and rates of dissolving.