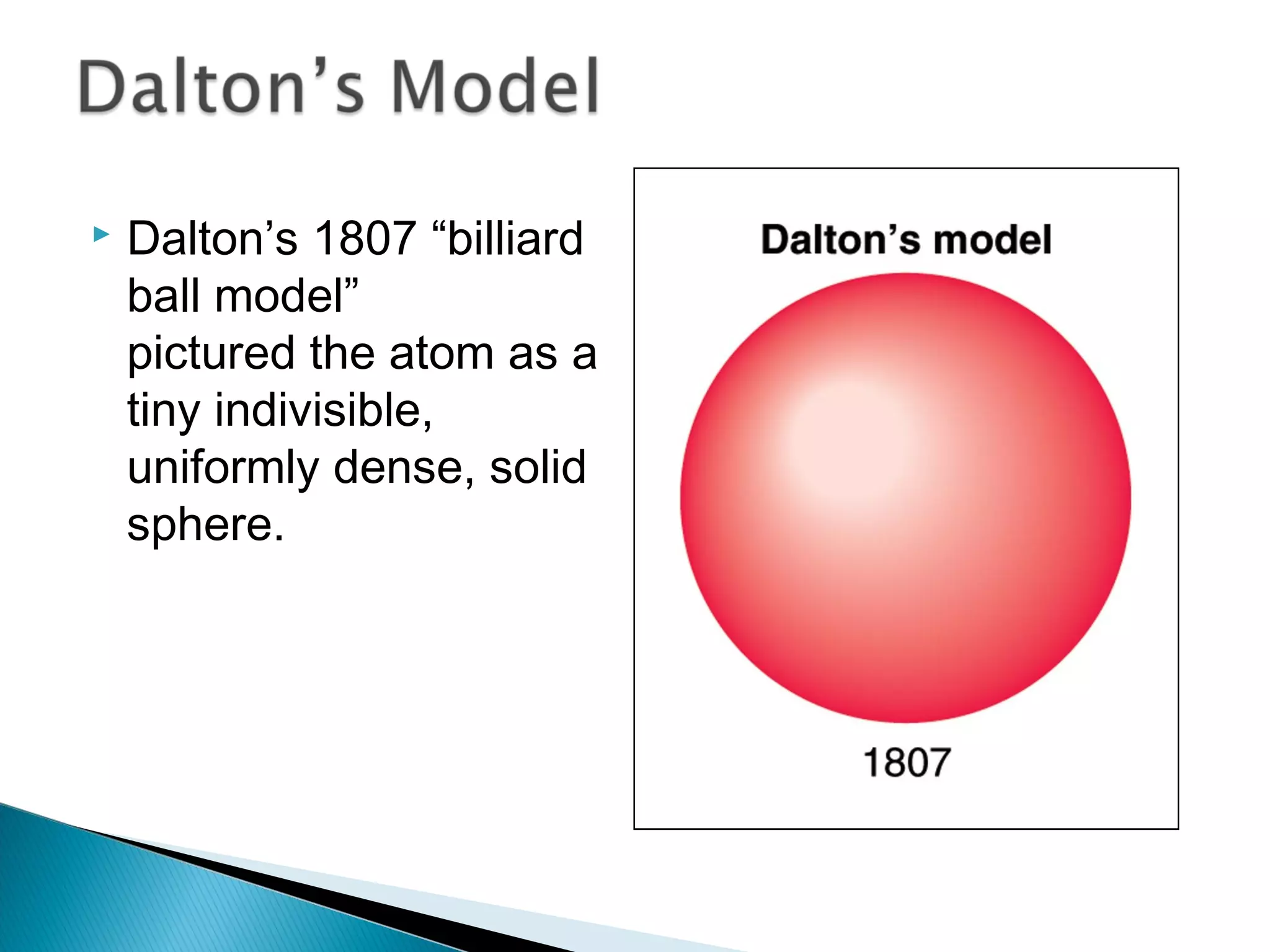
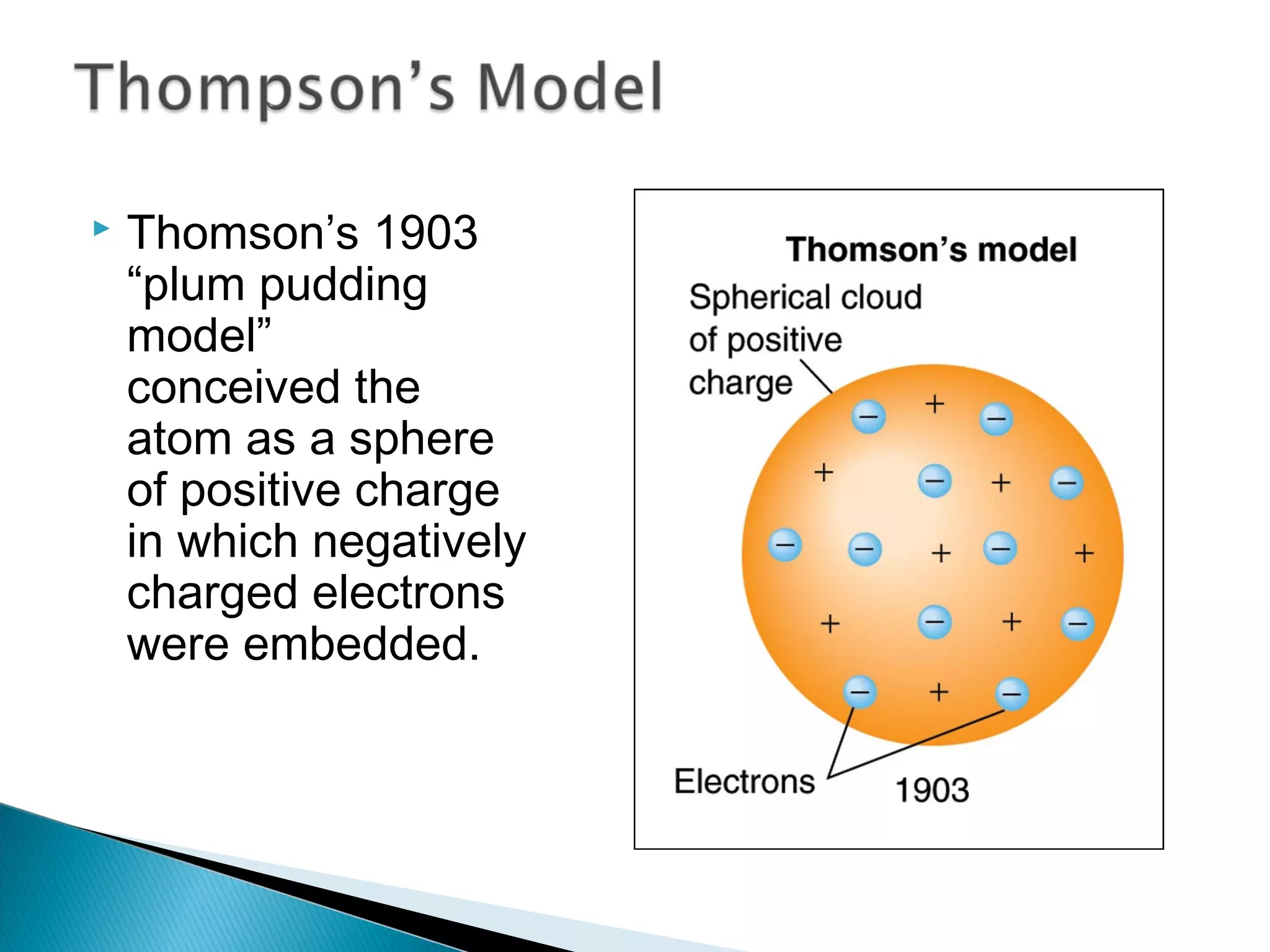
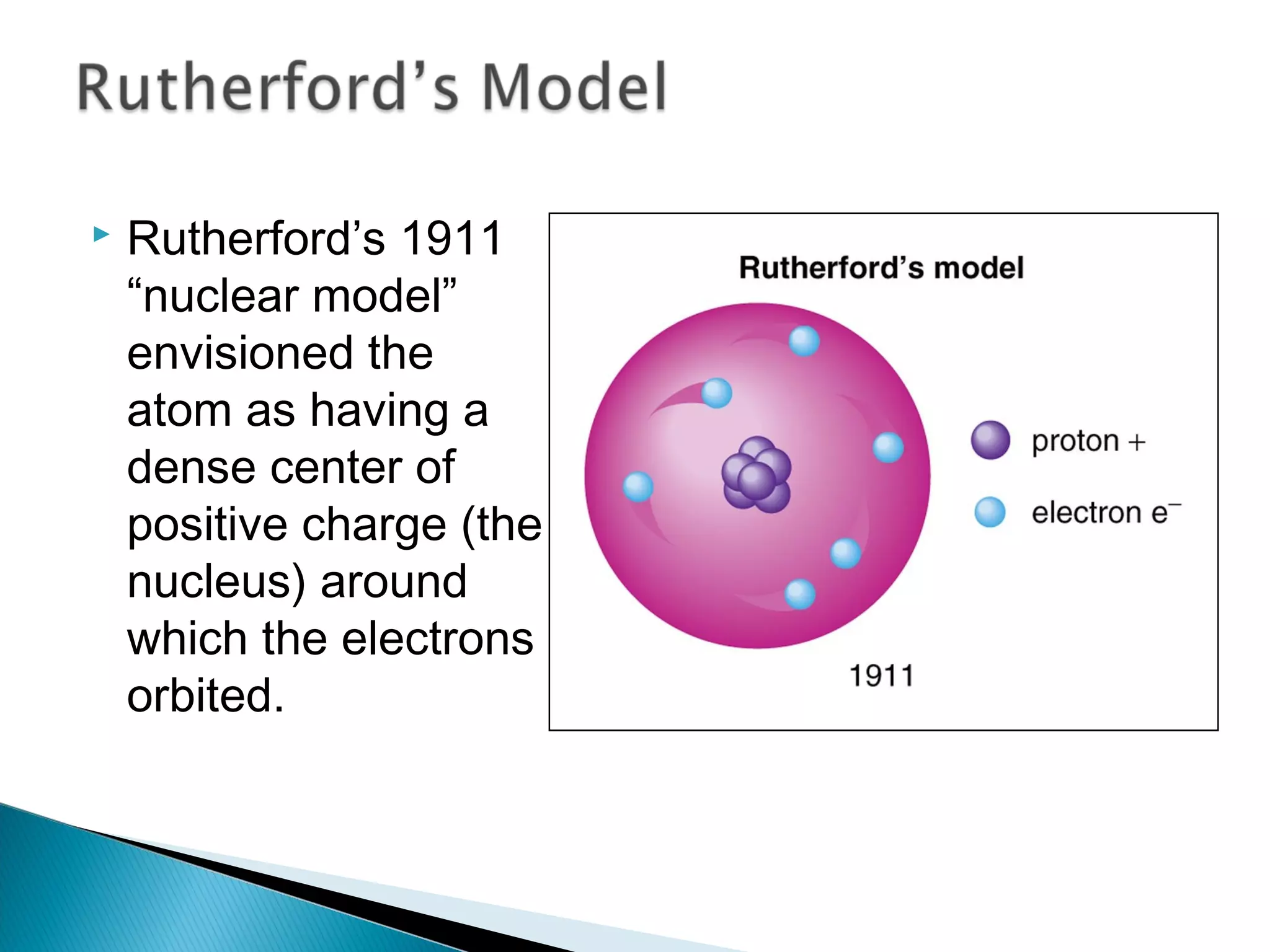
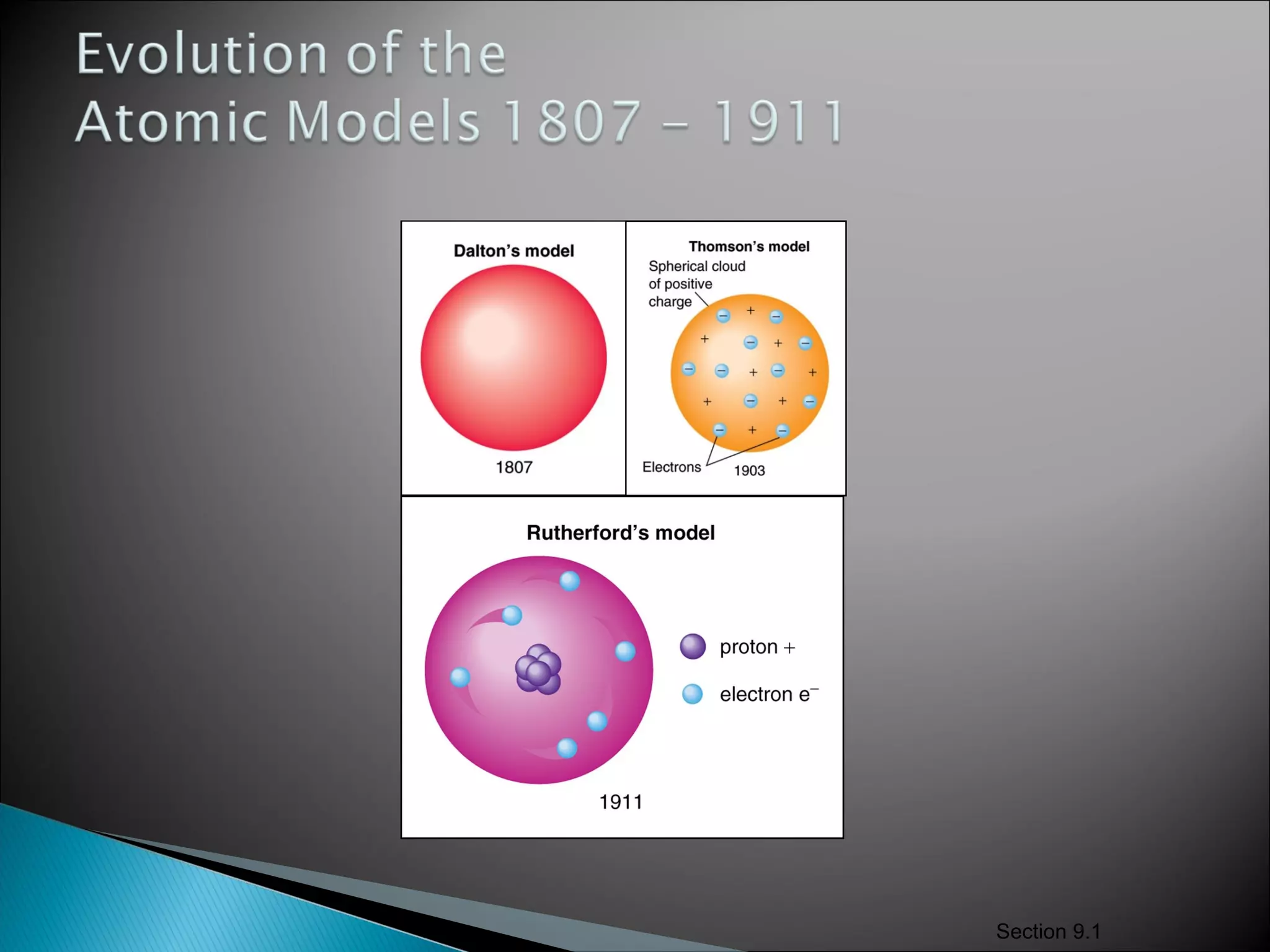
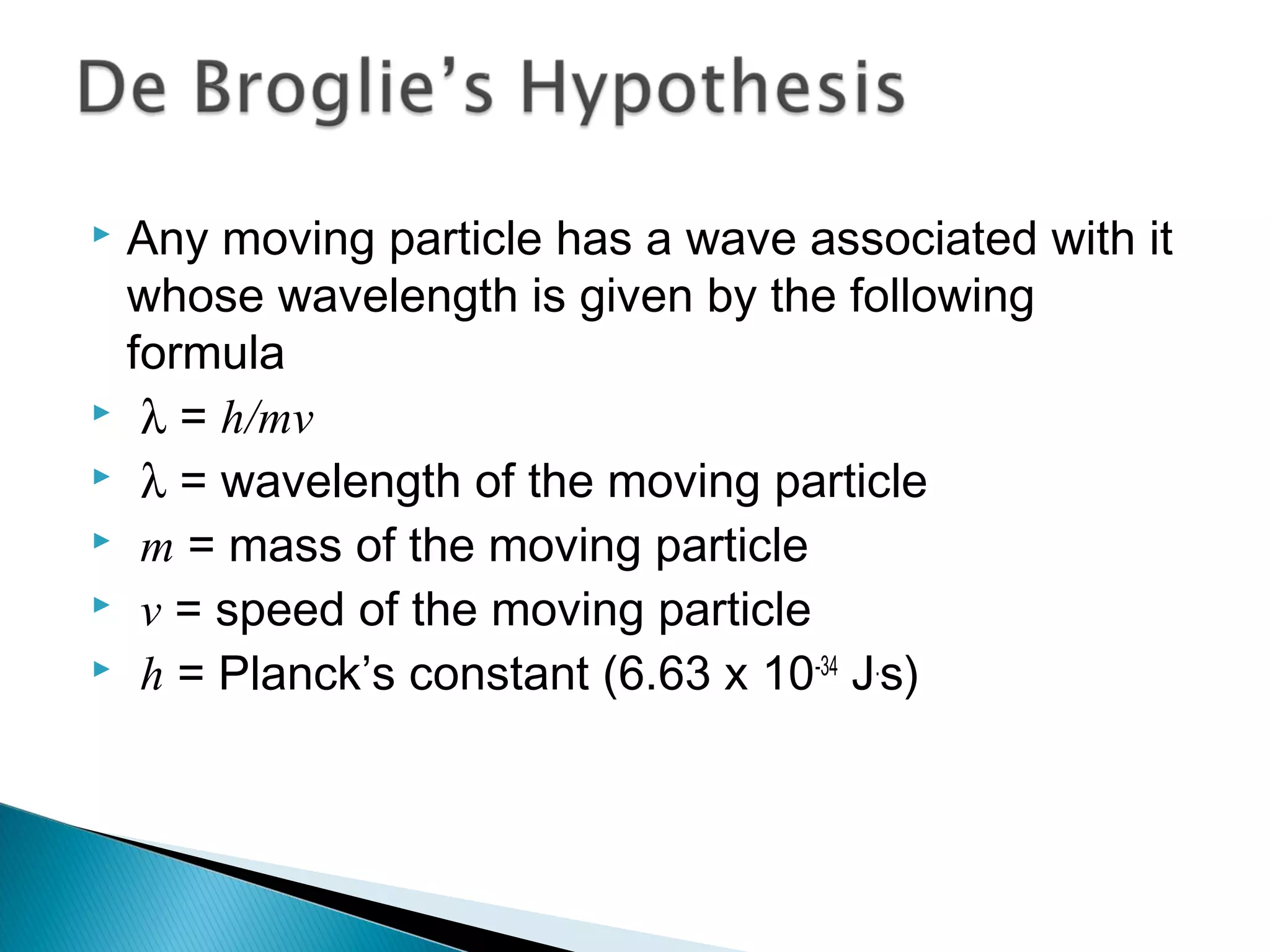
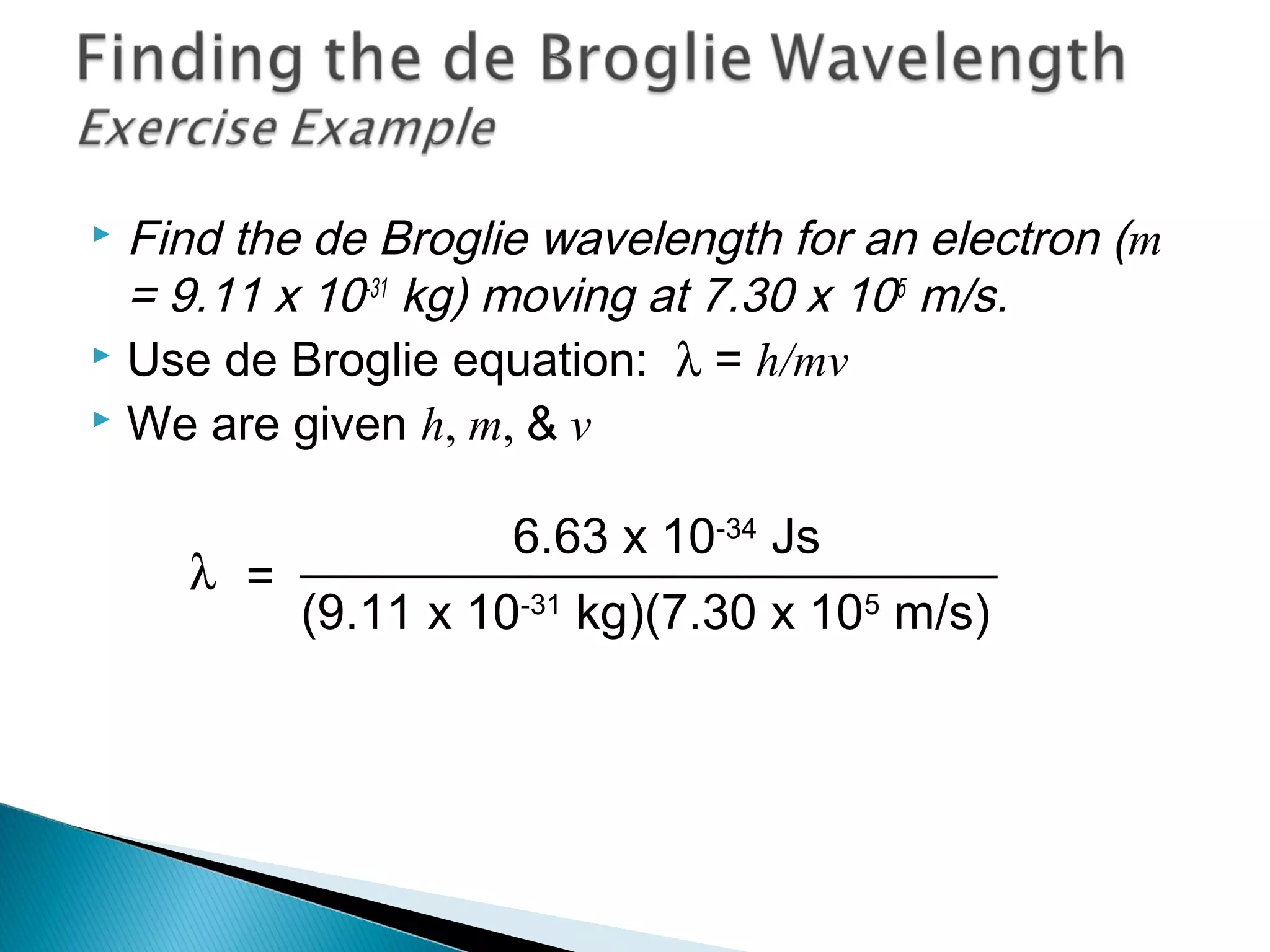
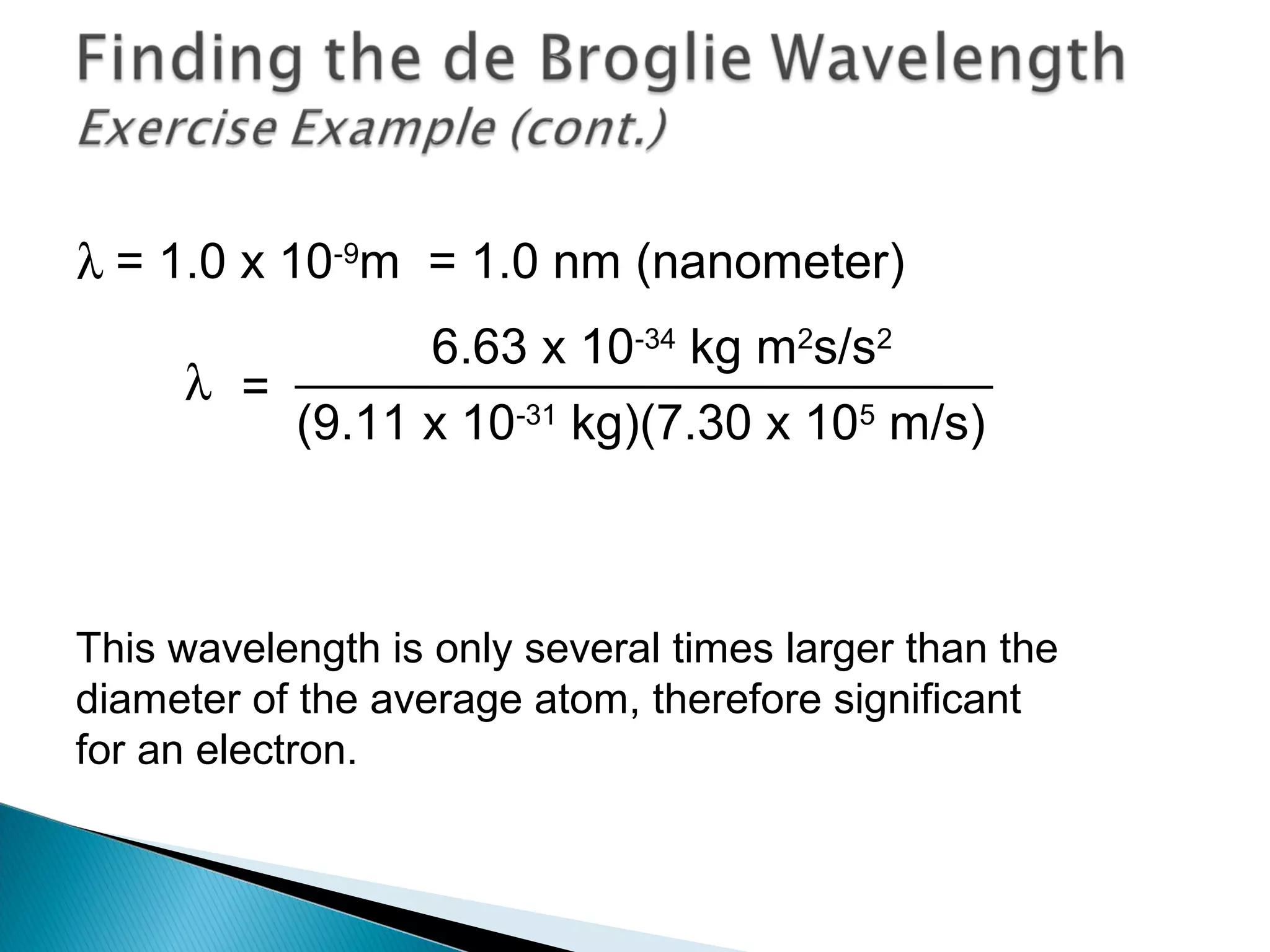
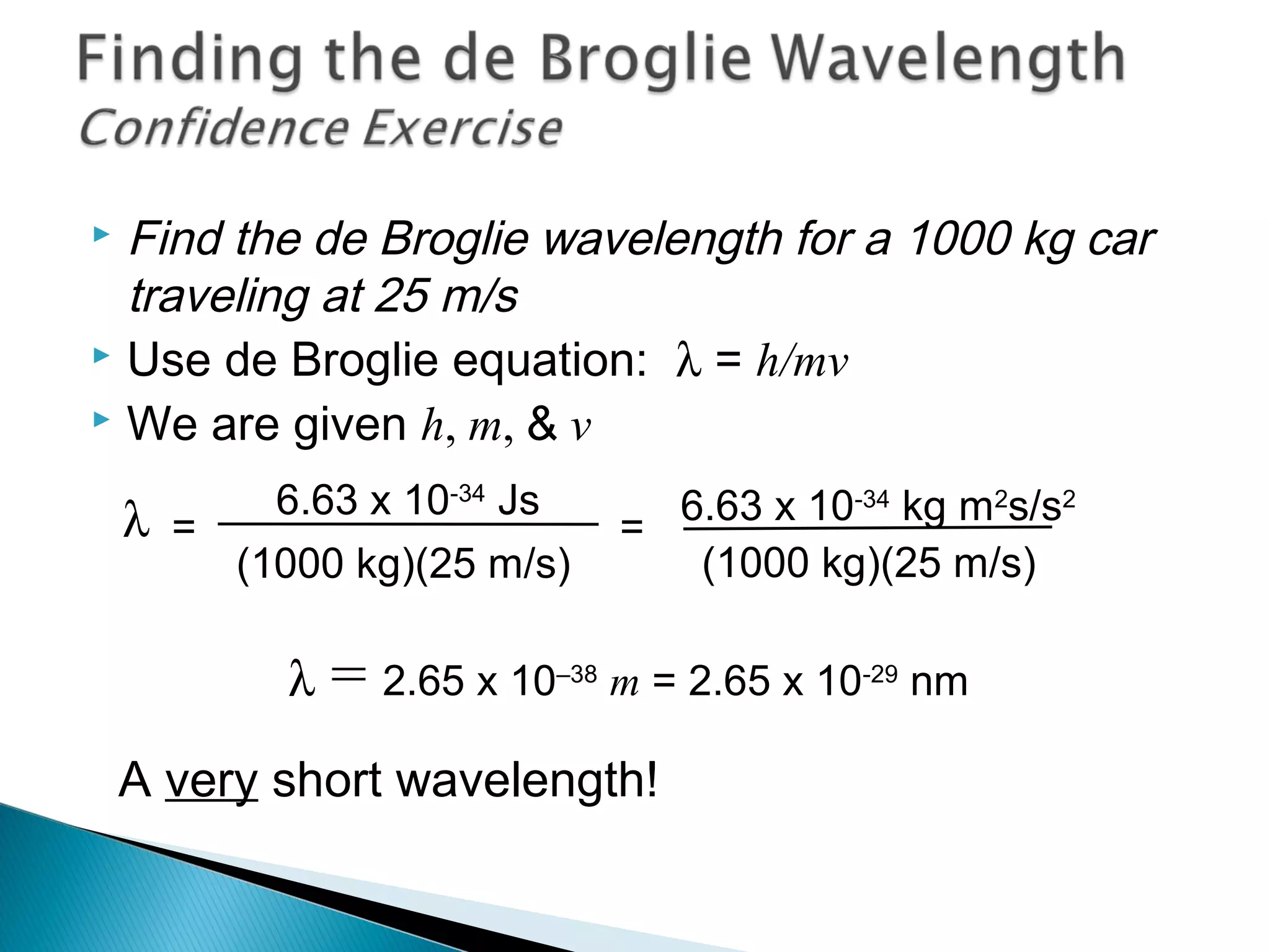
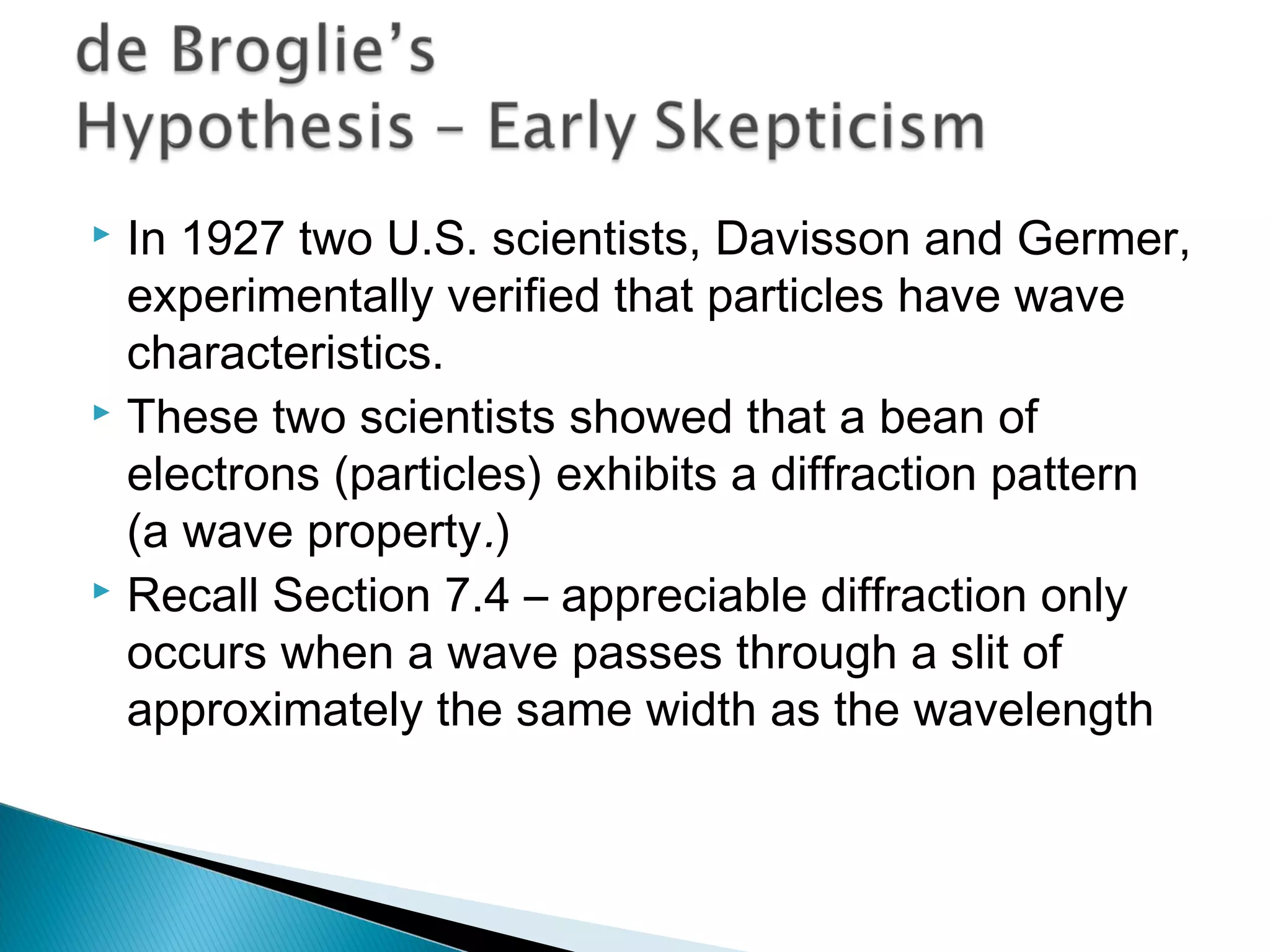
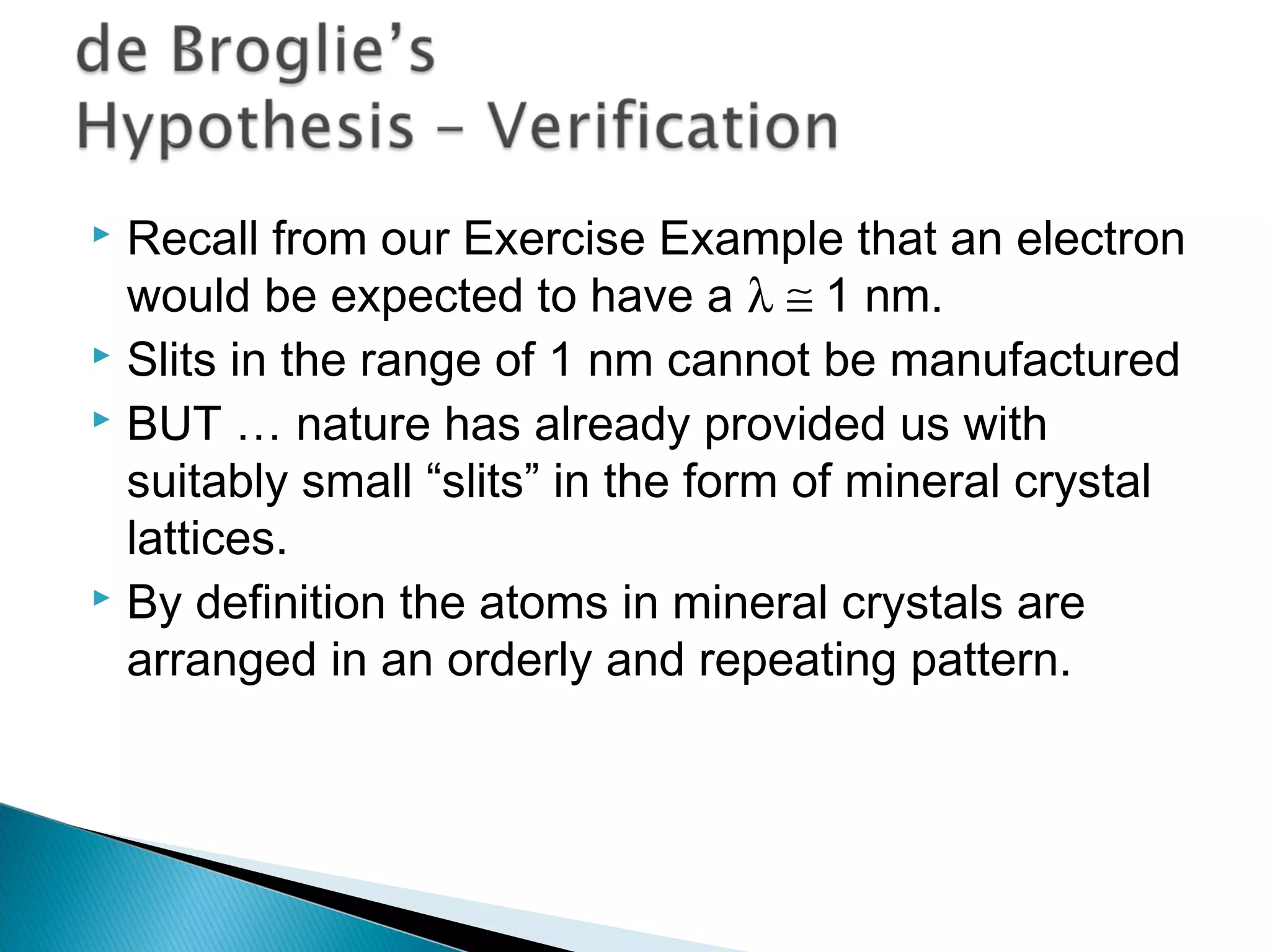
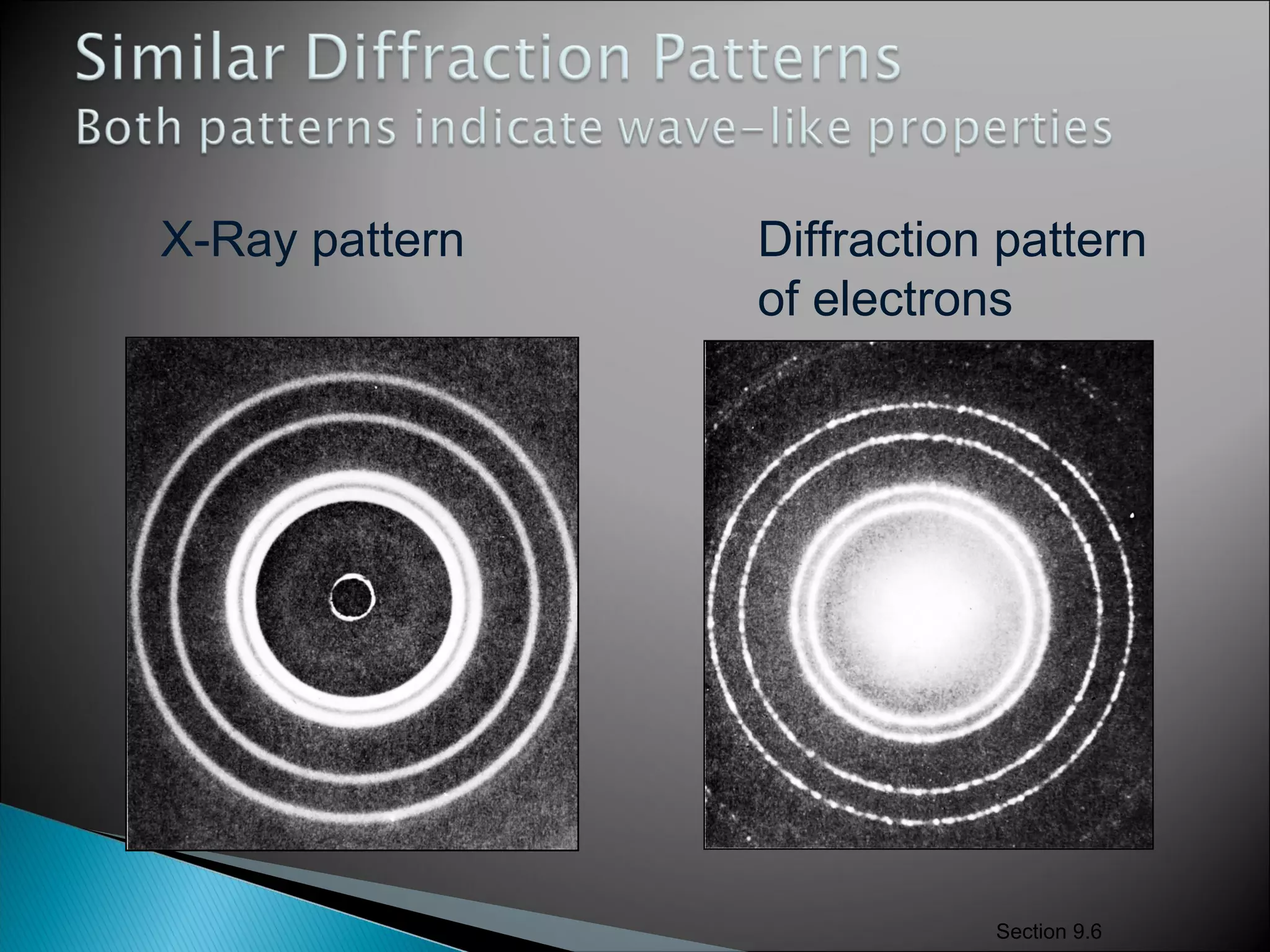
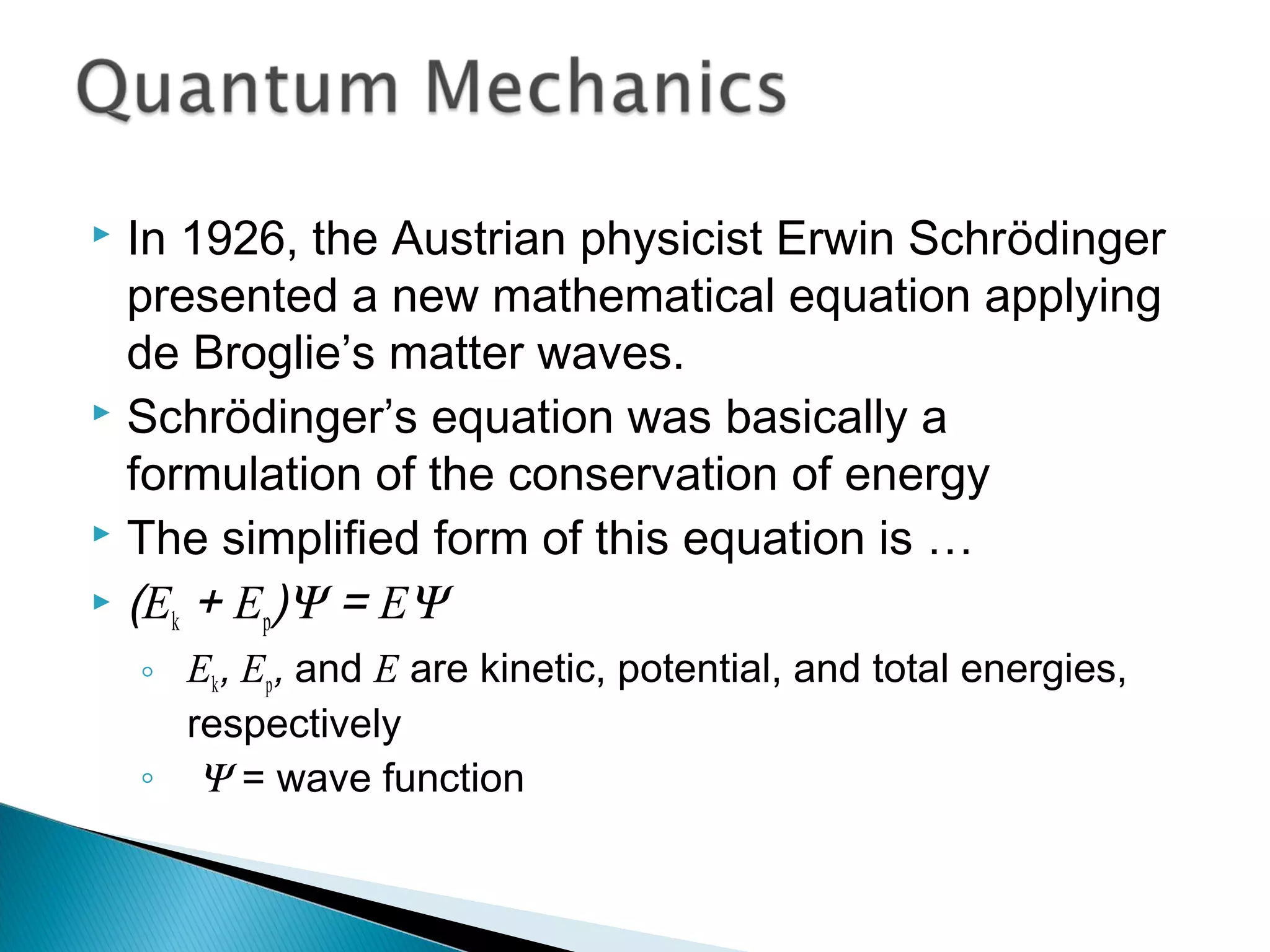
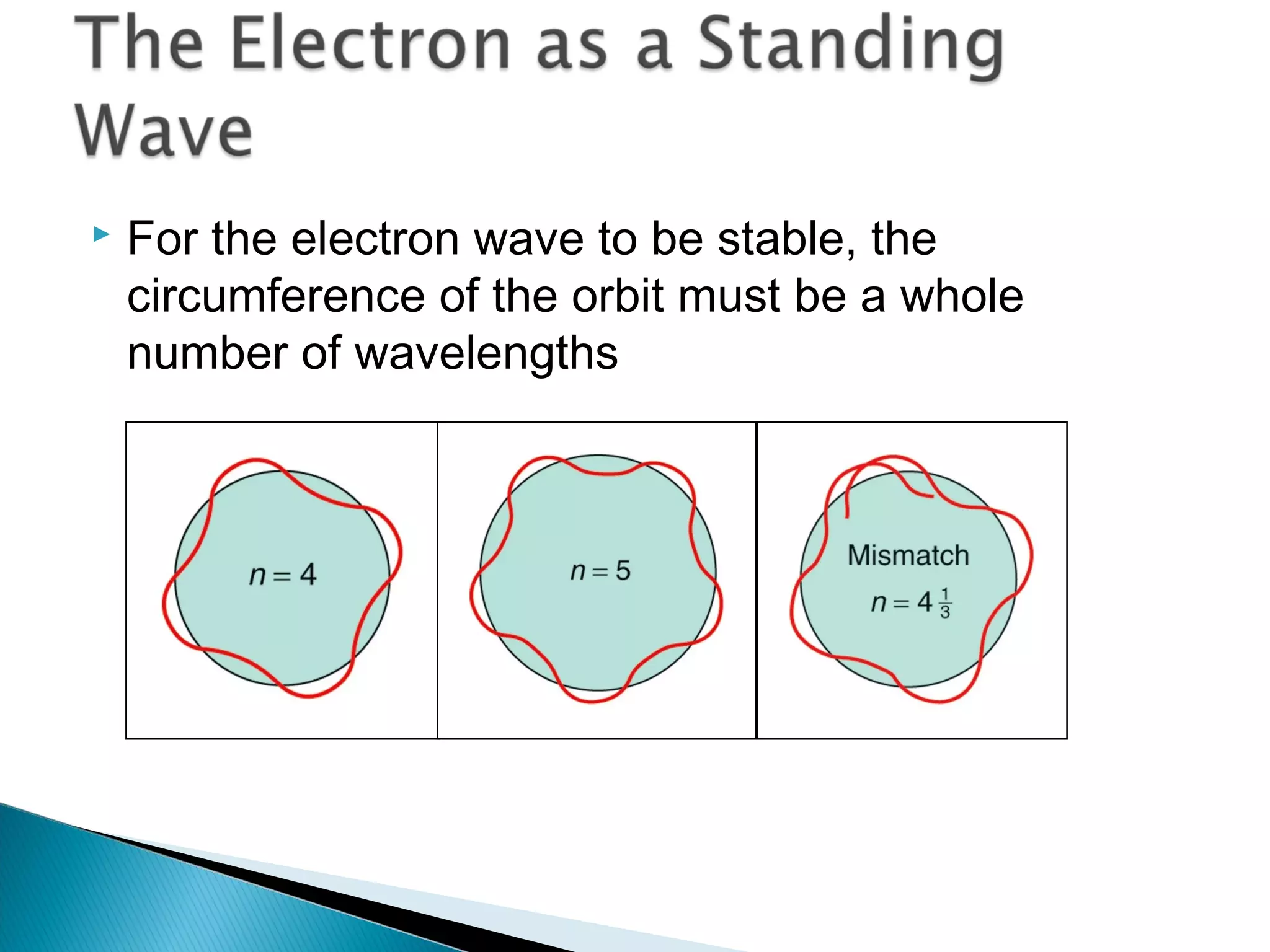
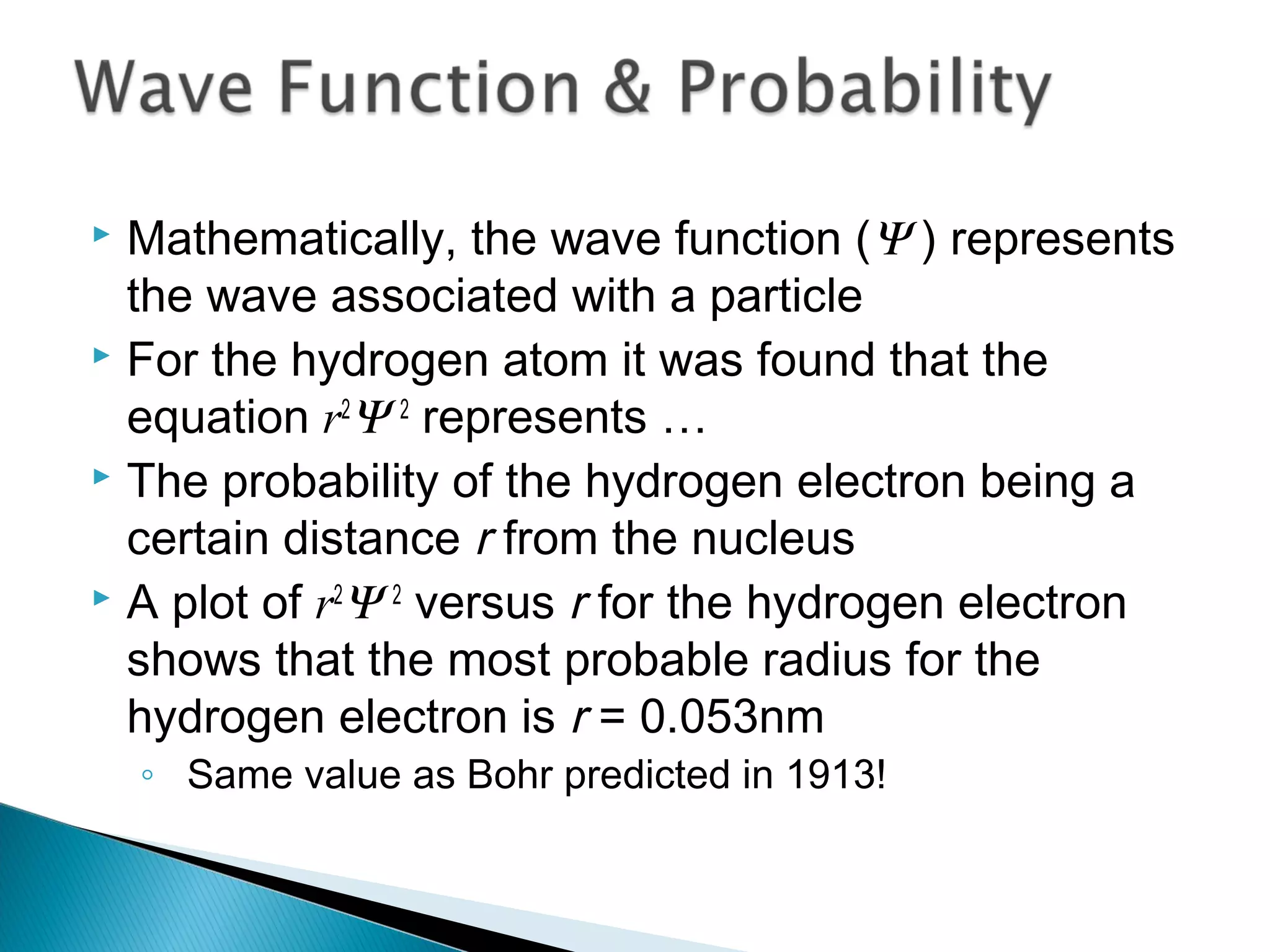
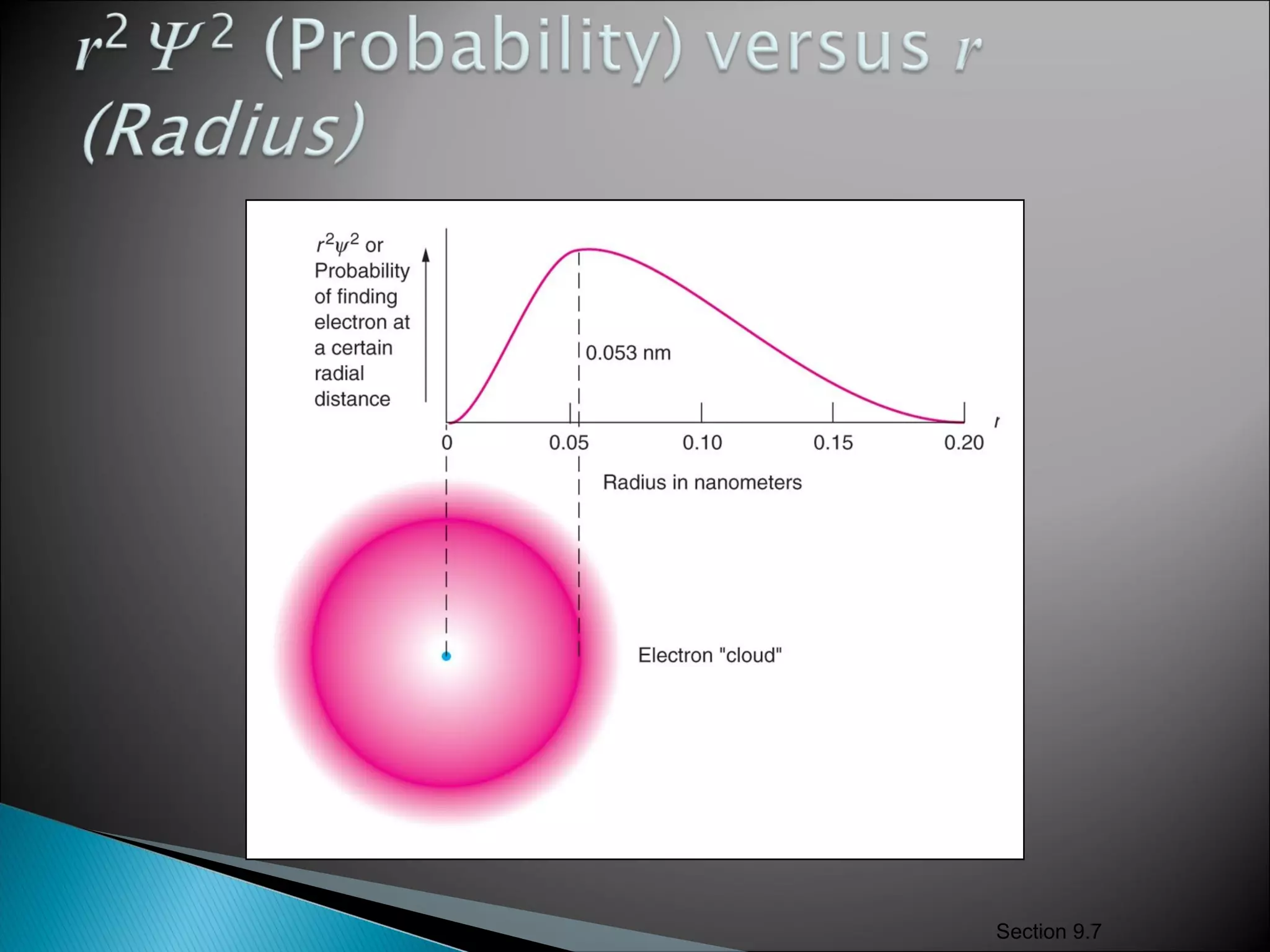
1) The document discusses the development of atomic physics models from Dalton's billiard ball model to Thomson's plum pudding model to Rutherford's nuclear model.
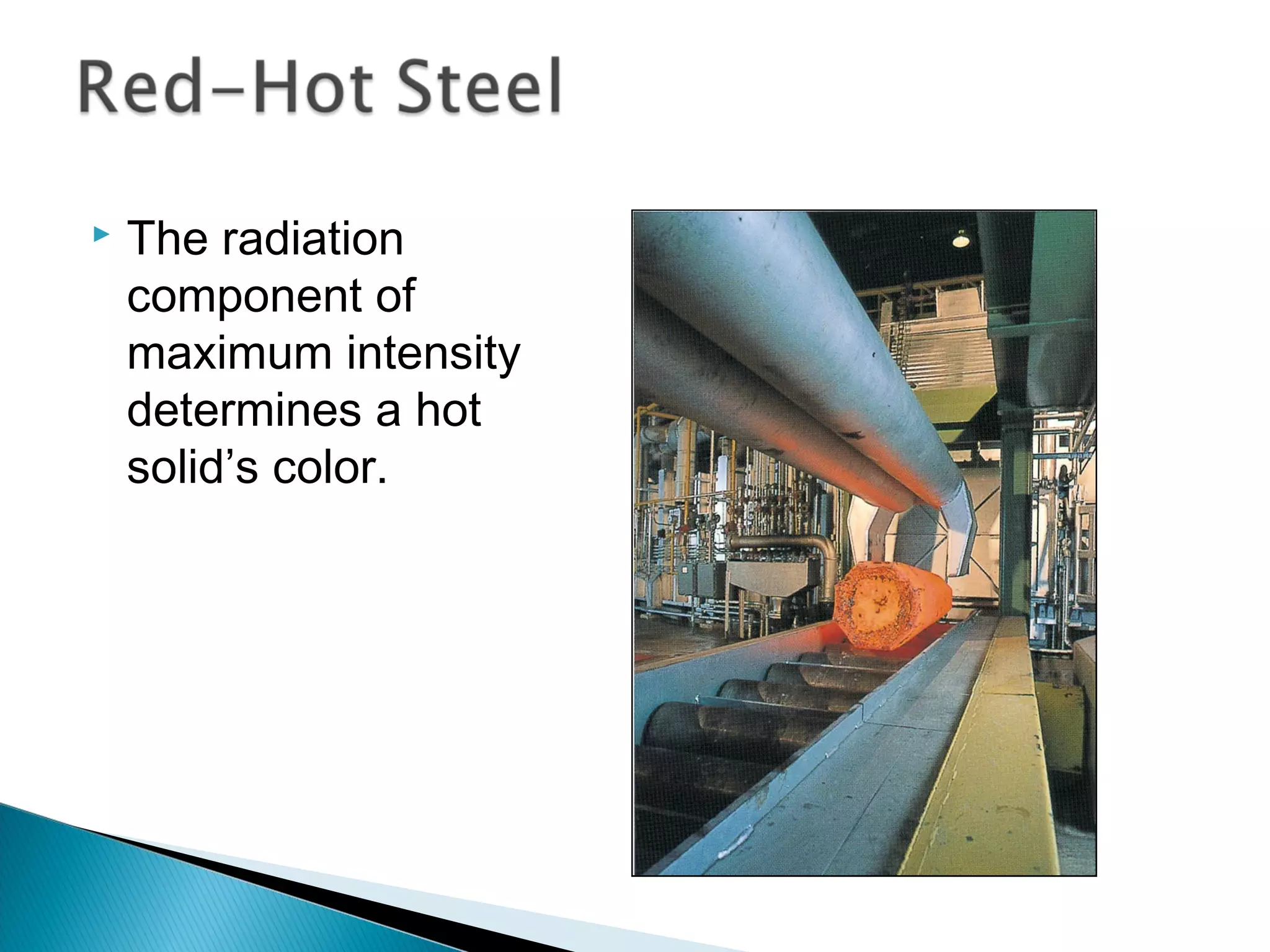
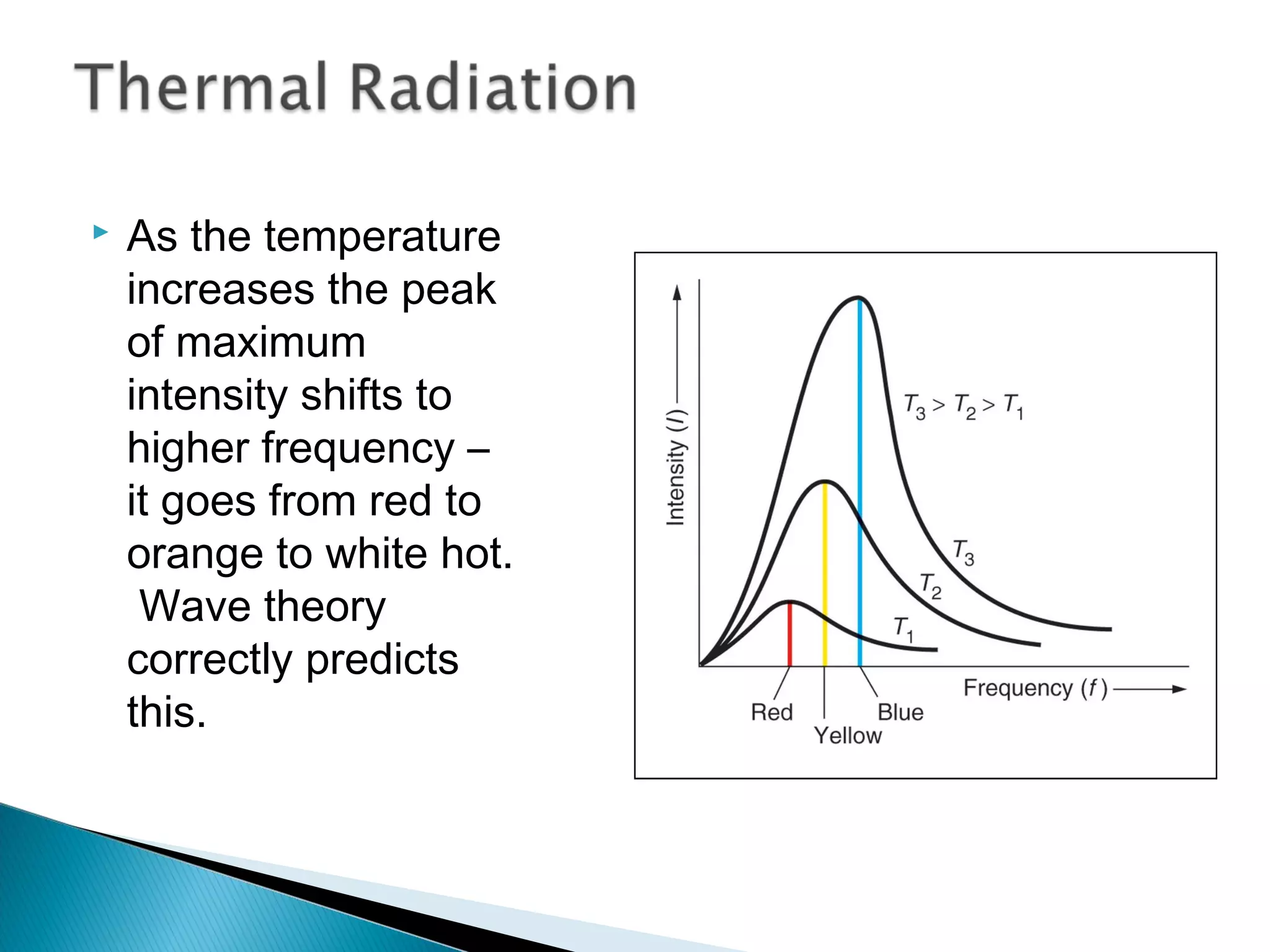
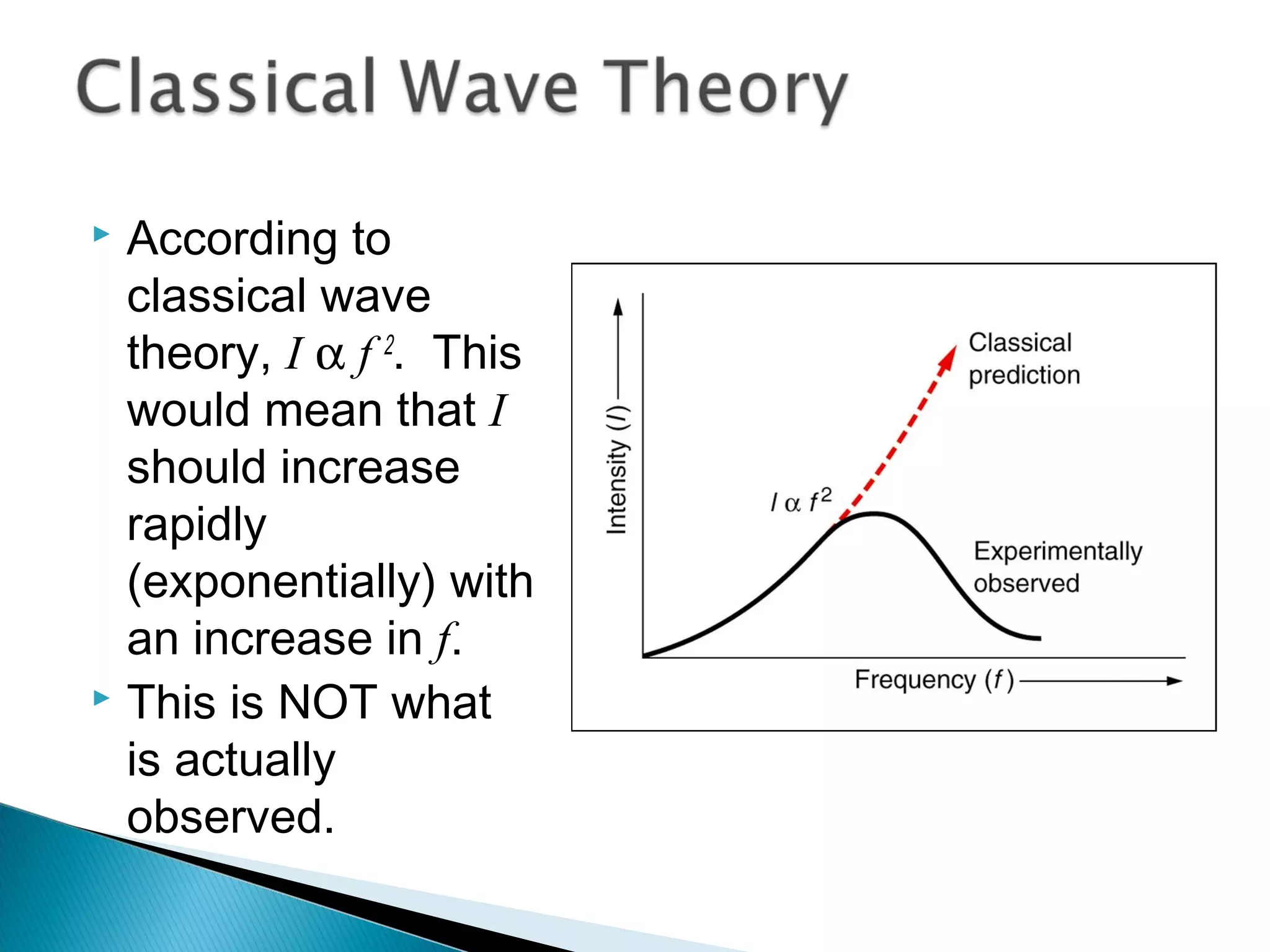
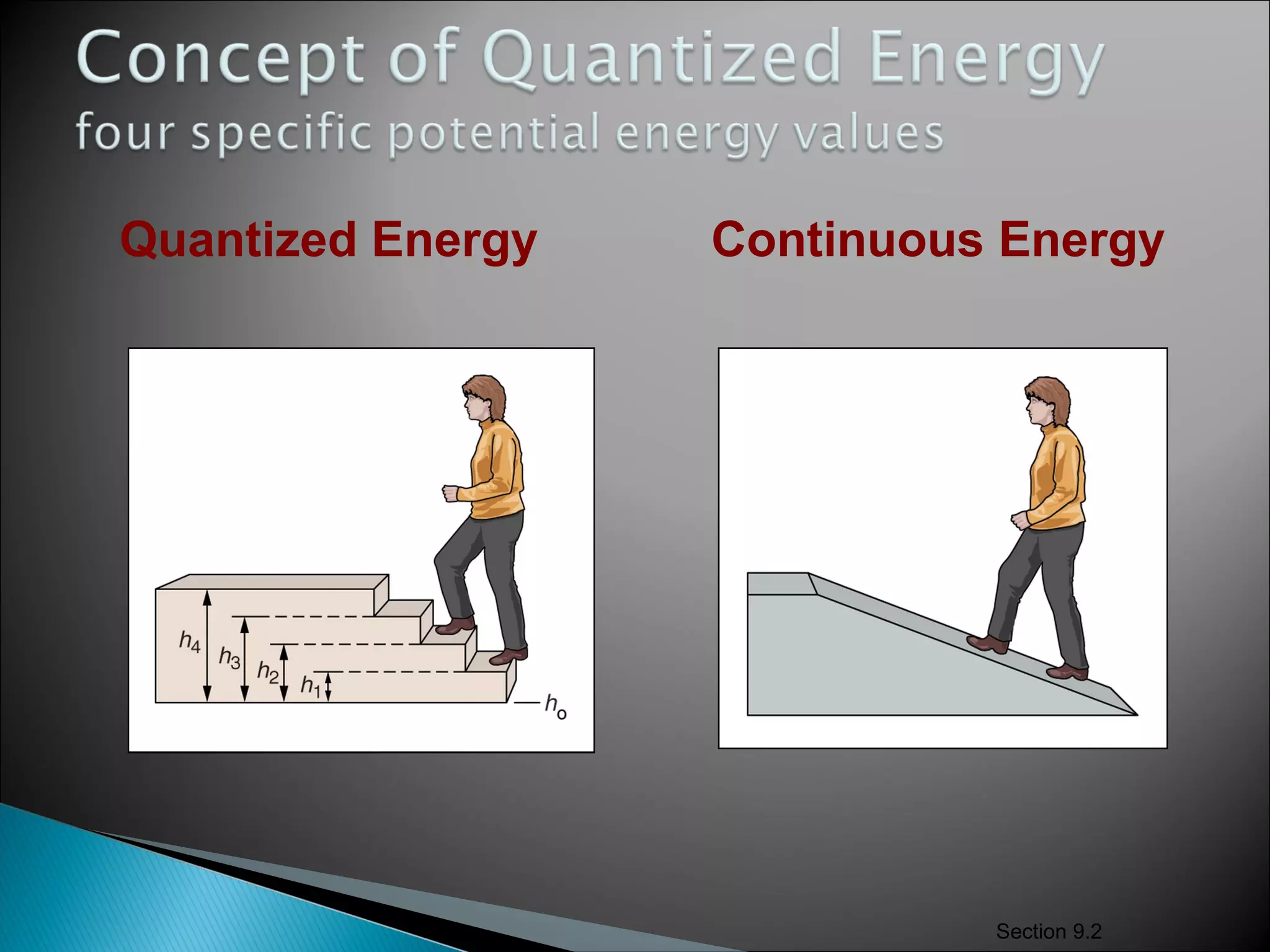
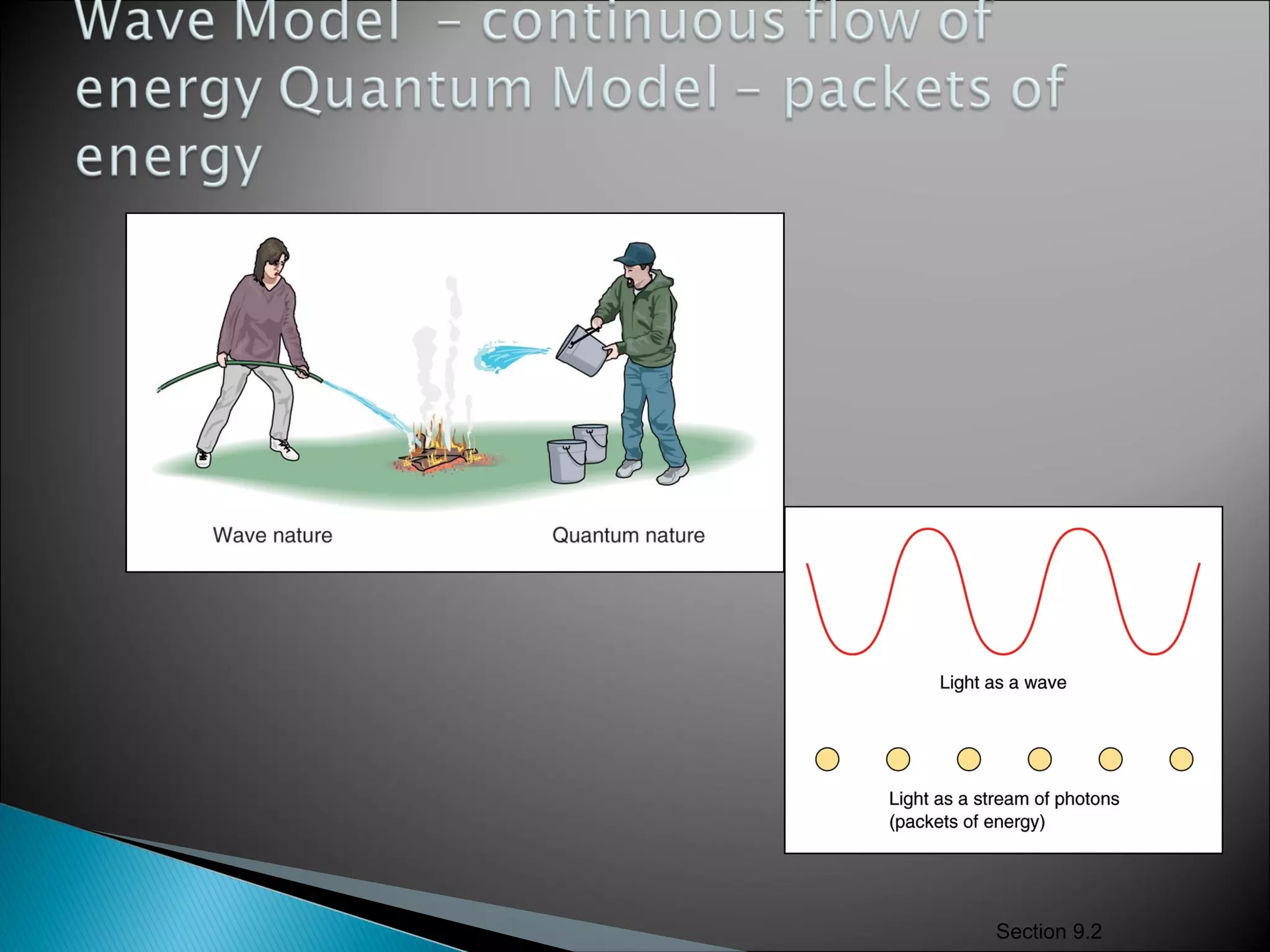
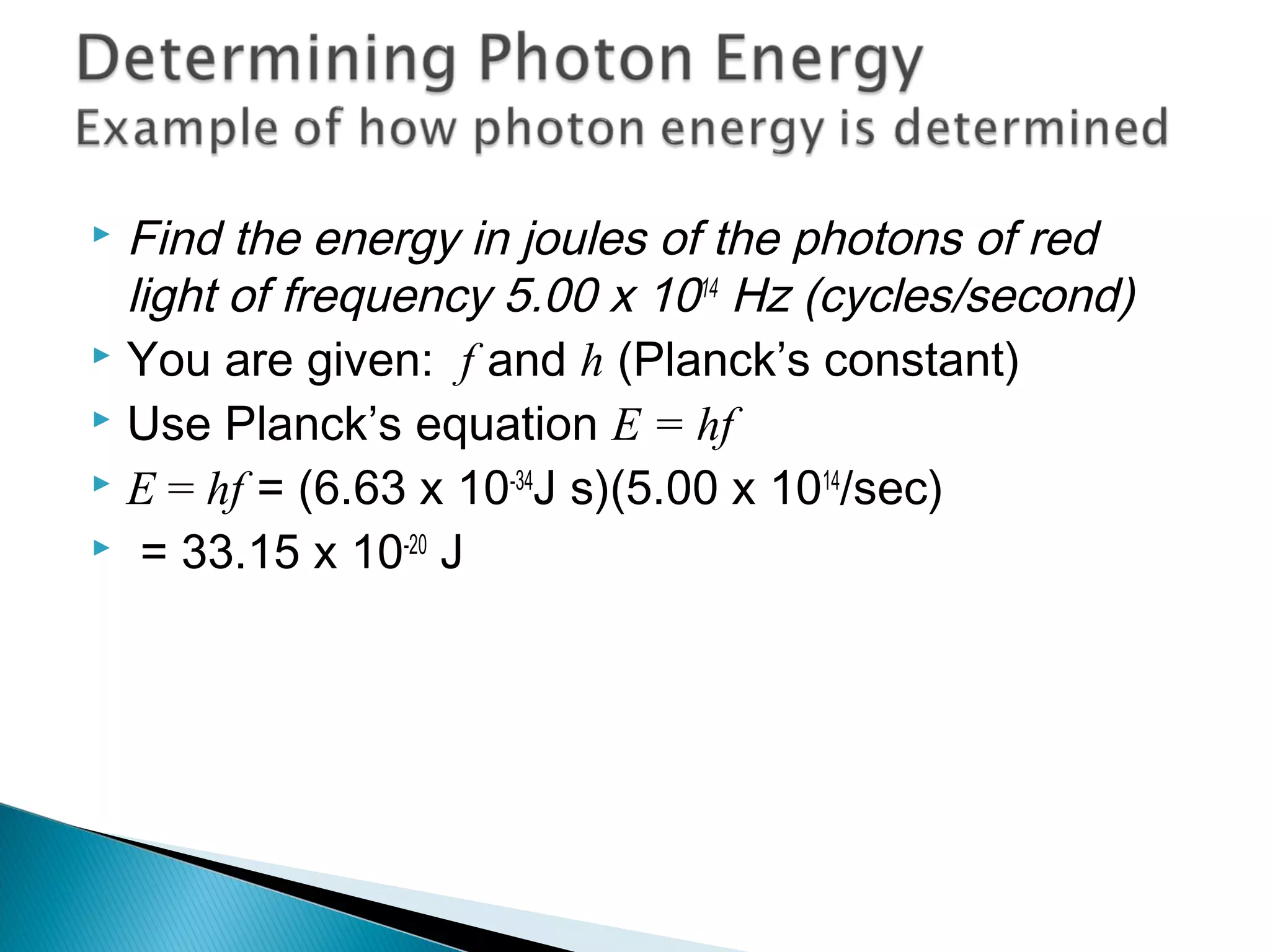
2) It then explains Planck's solution to the ultraviolet catastrophe by introducing the idea of quantized energy packets called quanta.
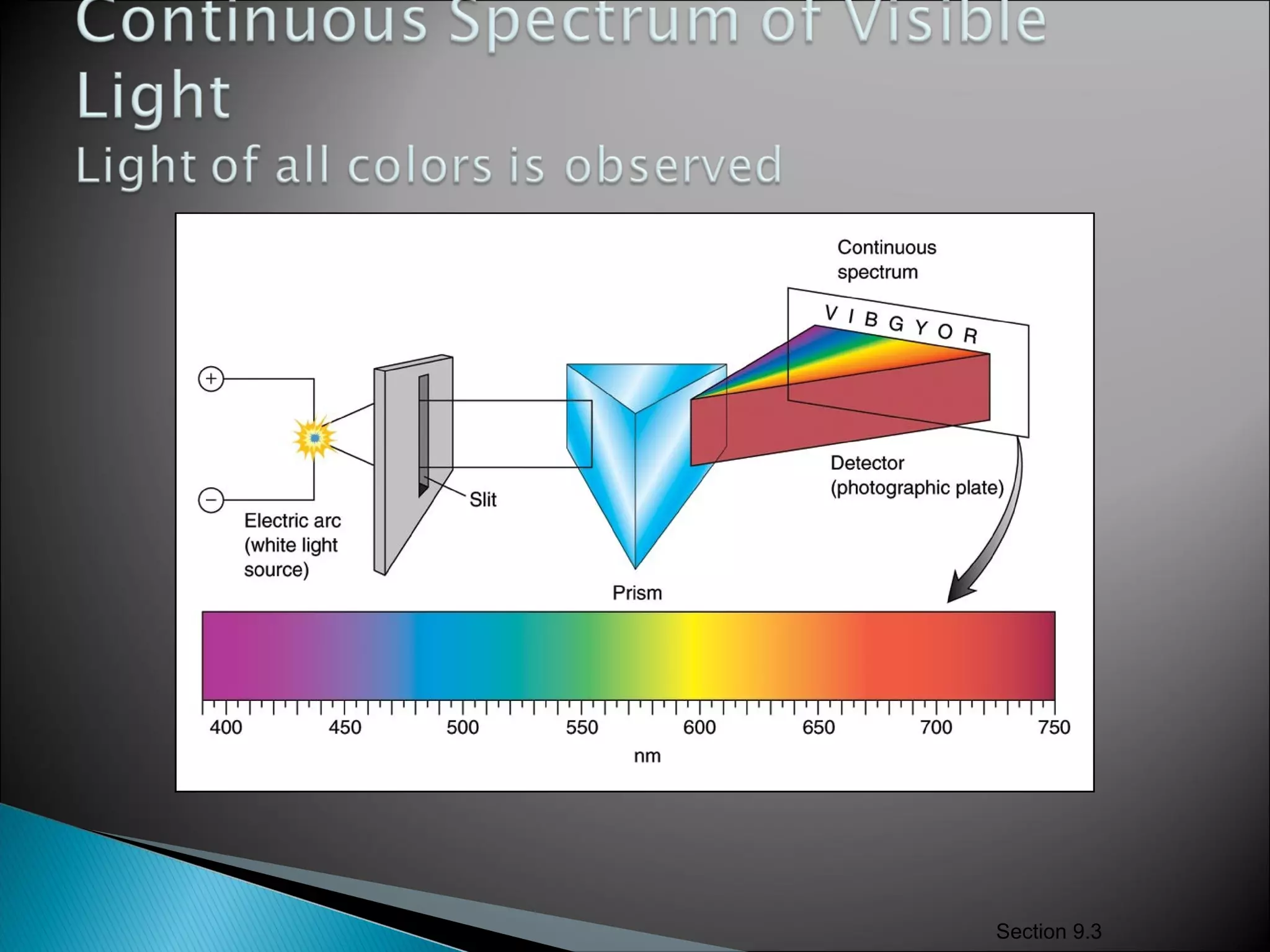
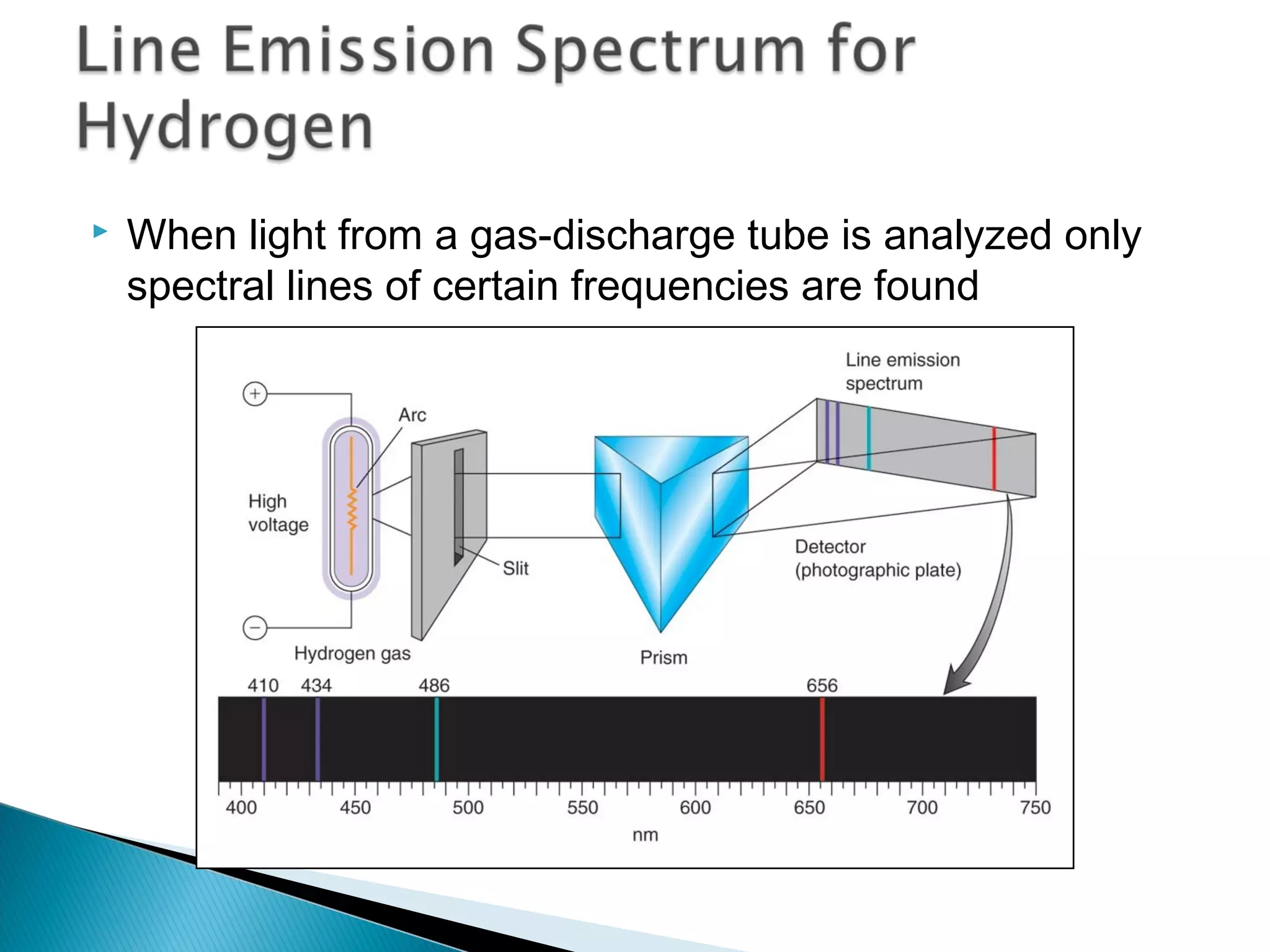
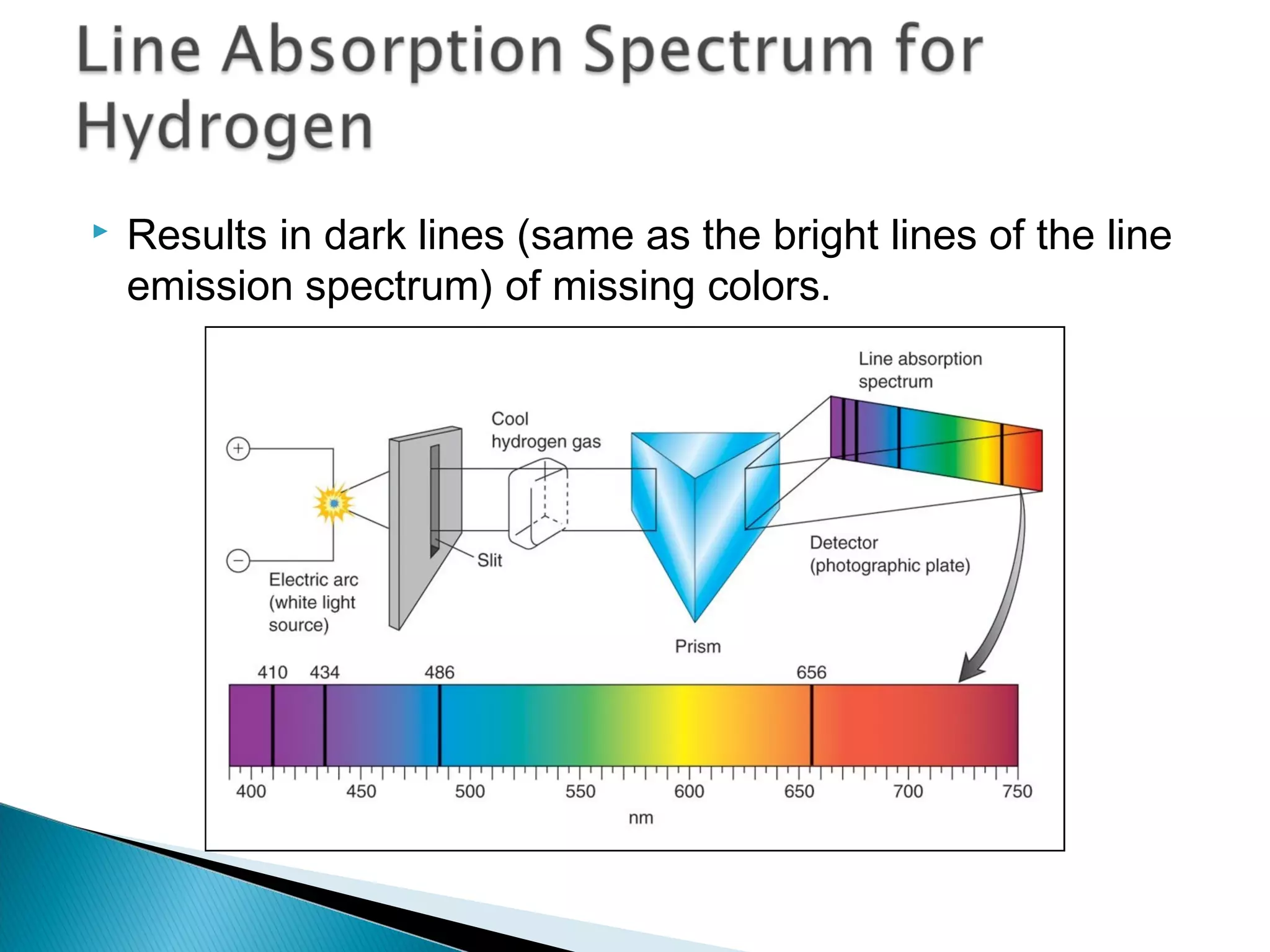
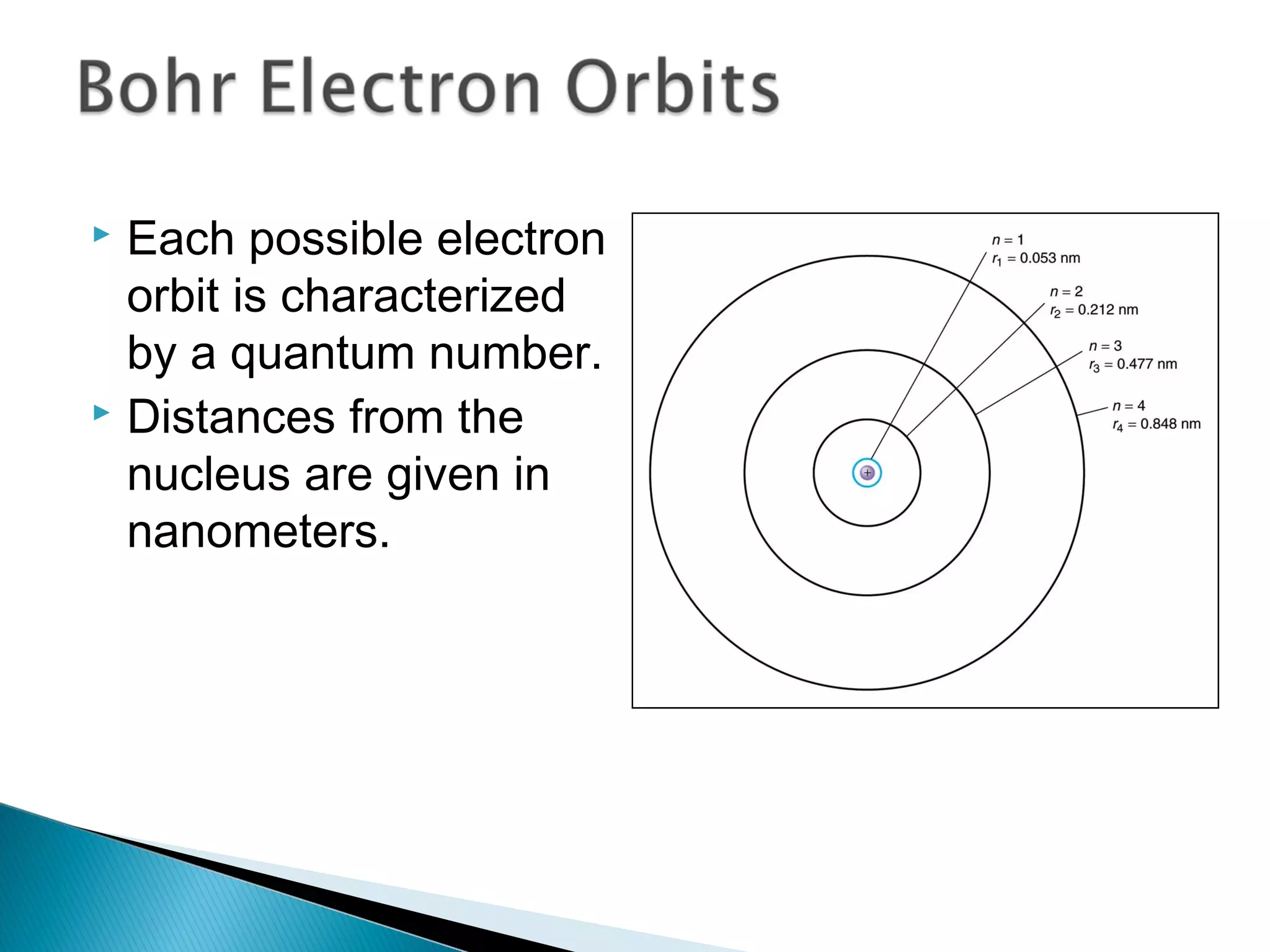
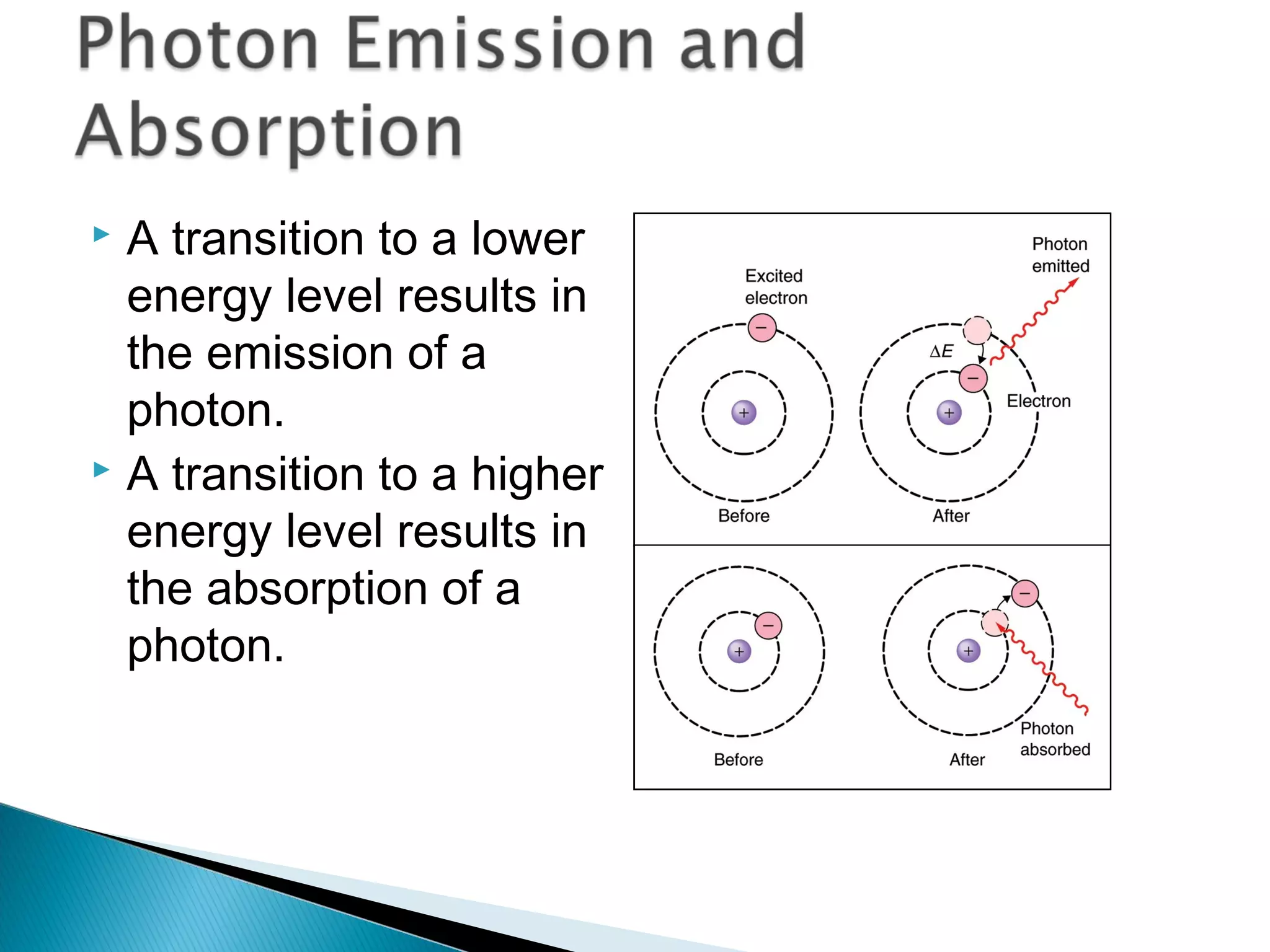
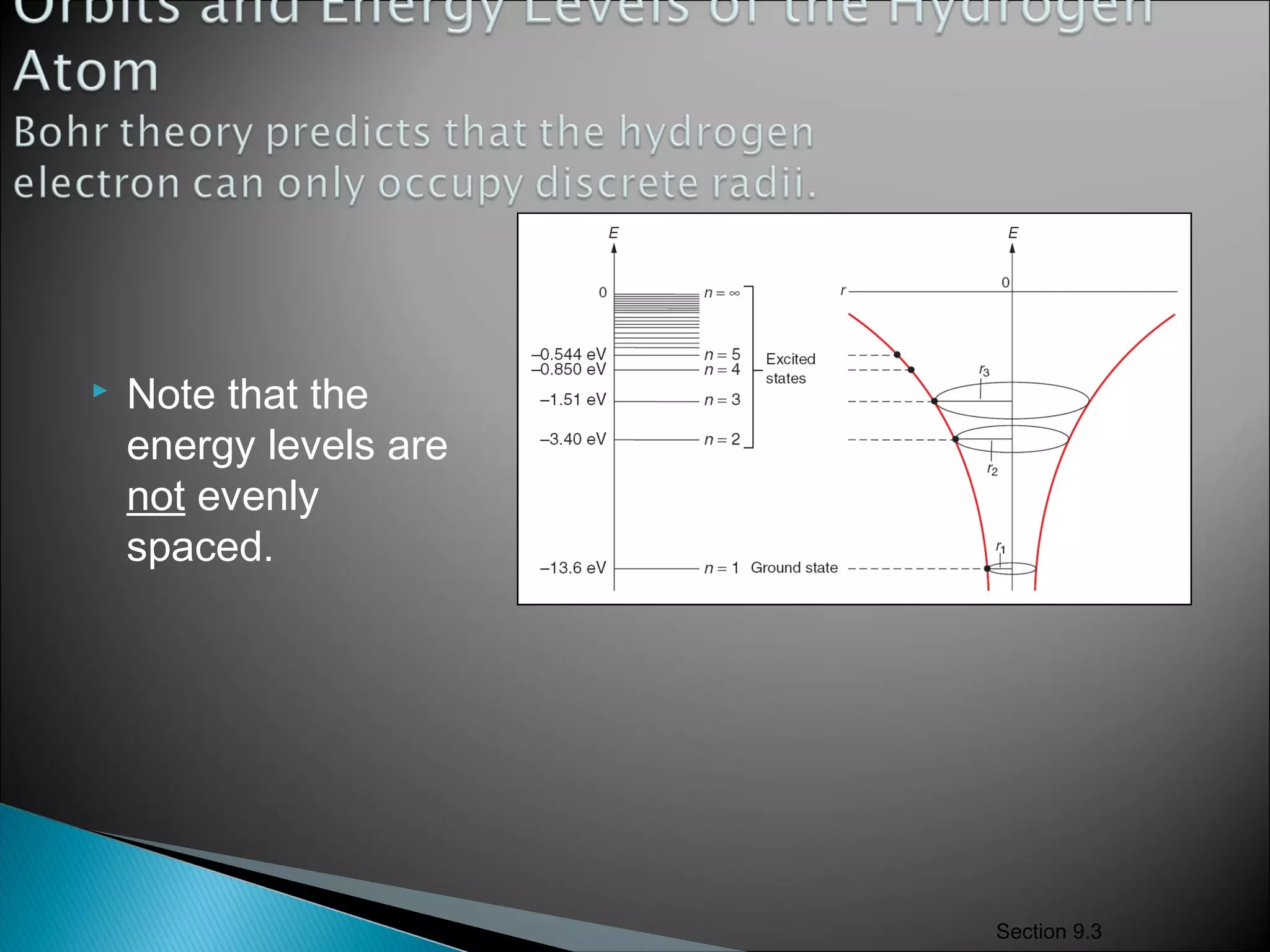
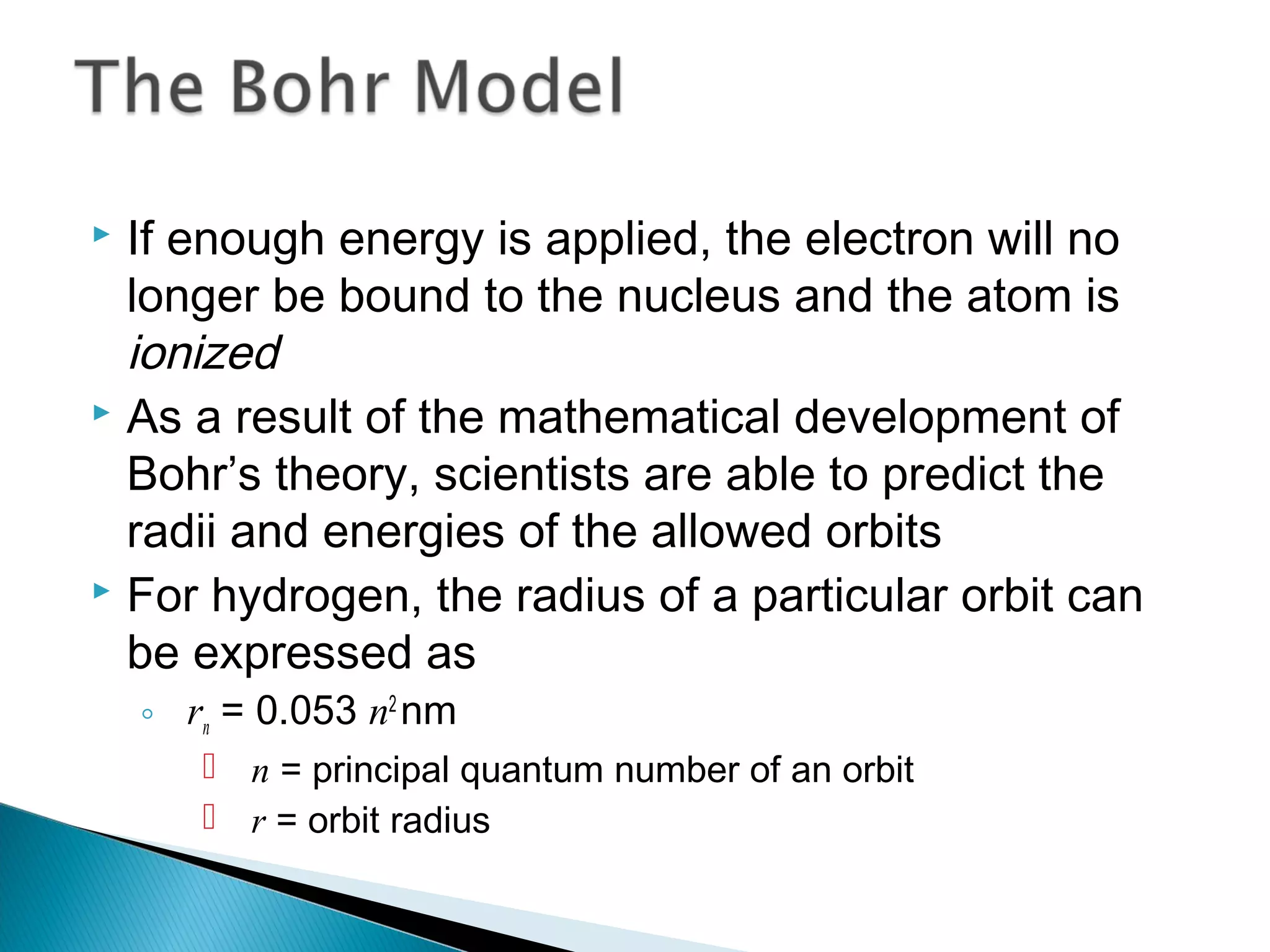
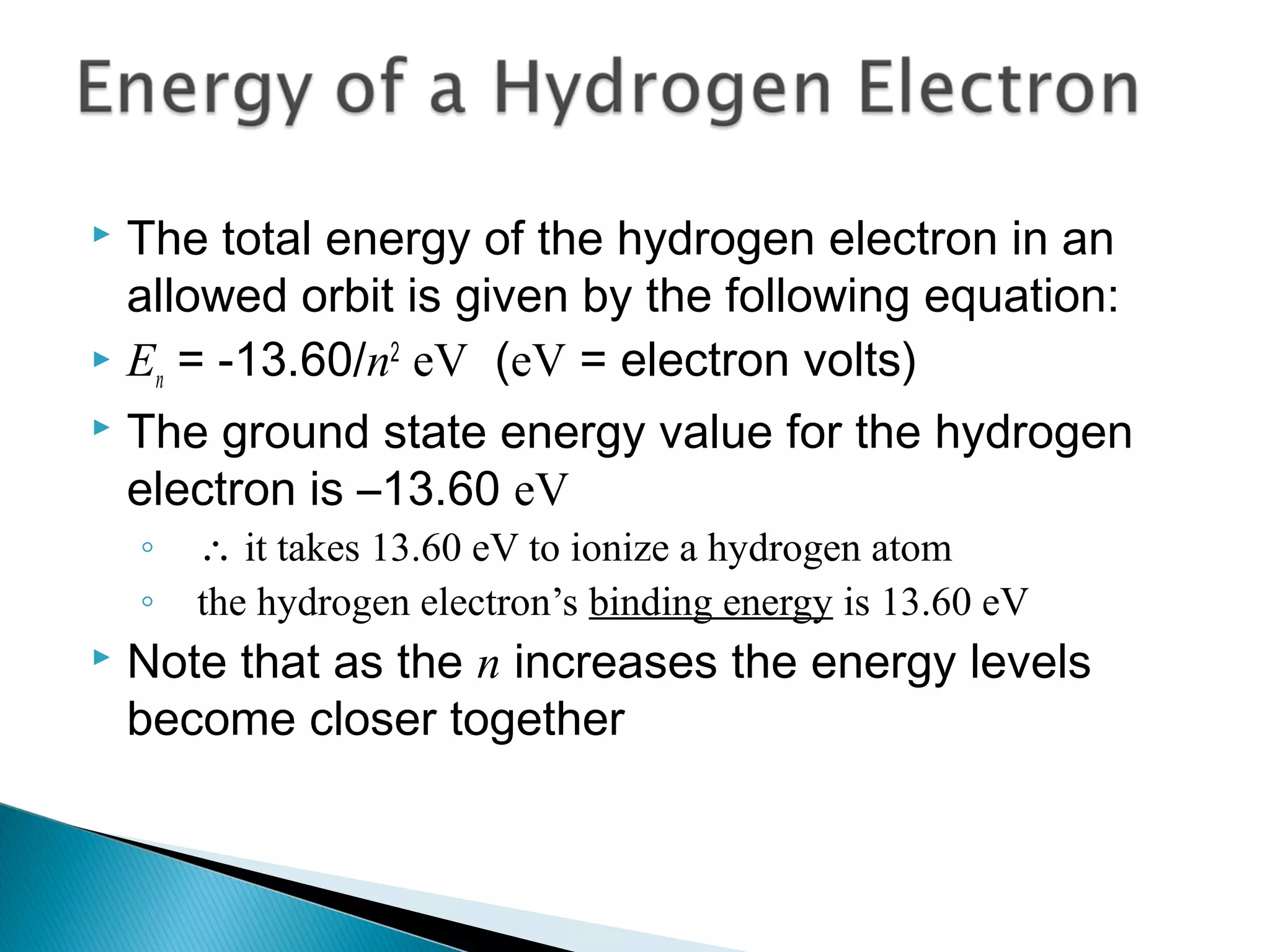
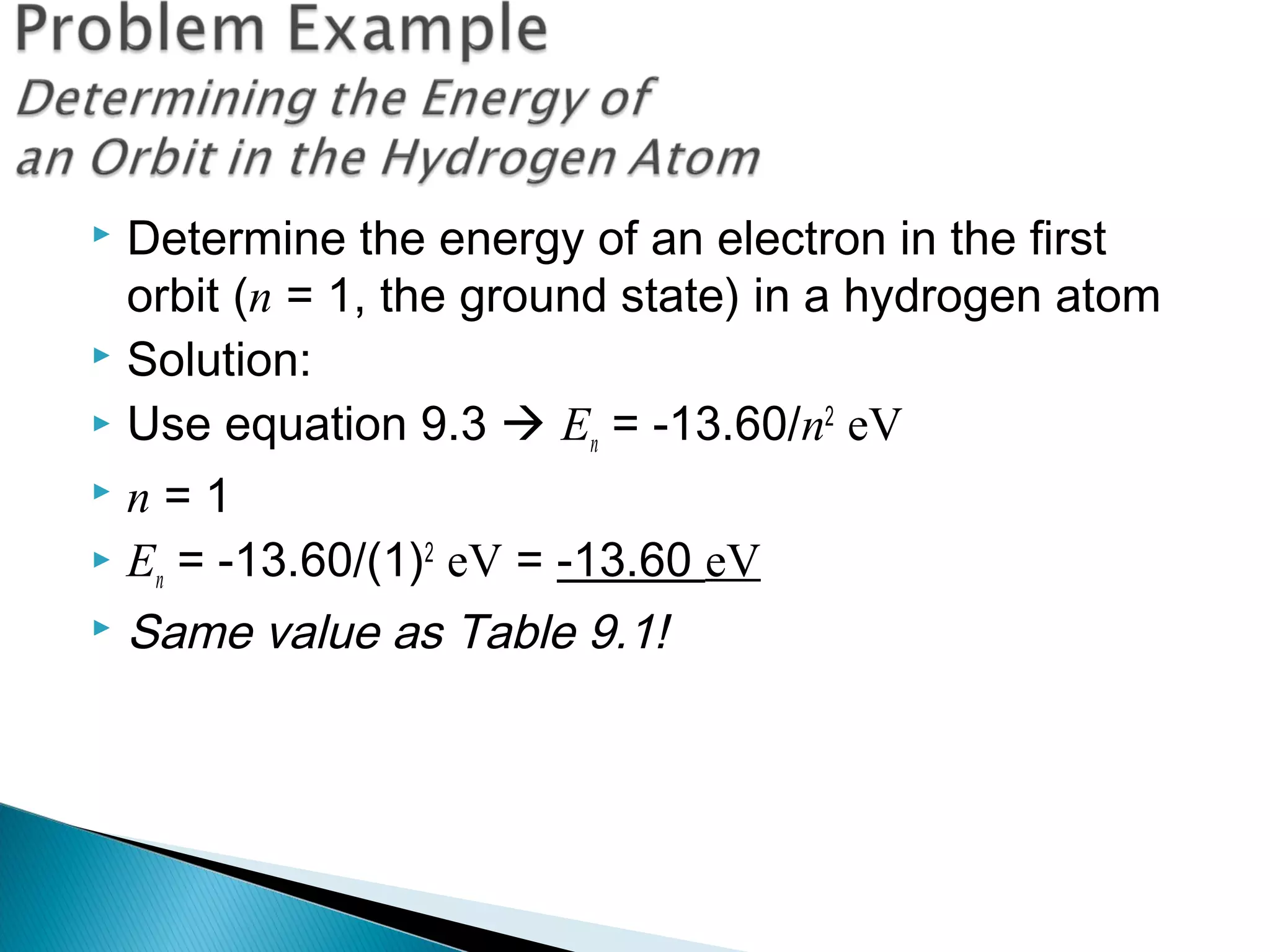
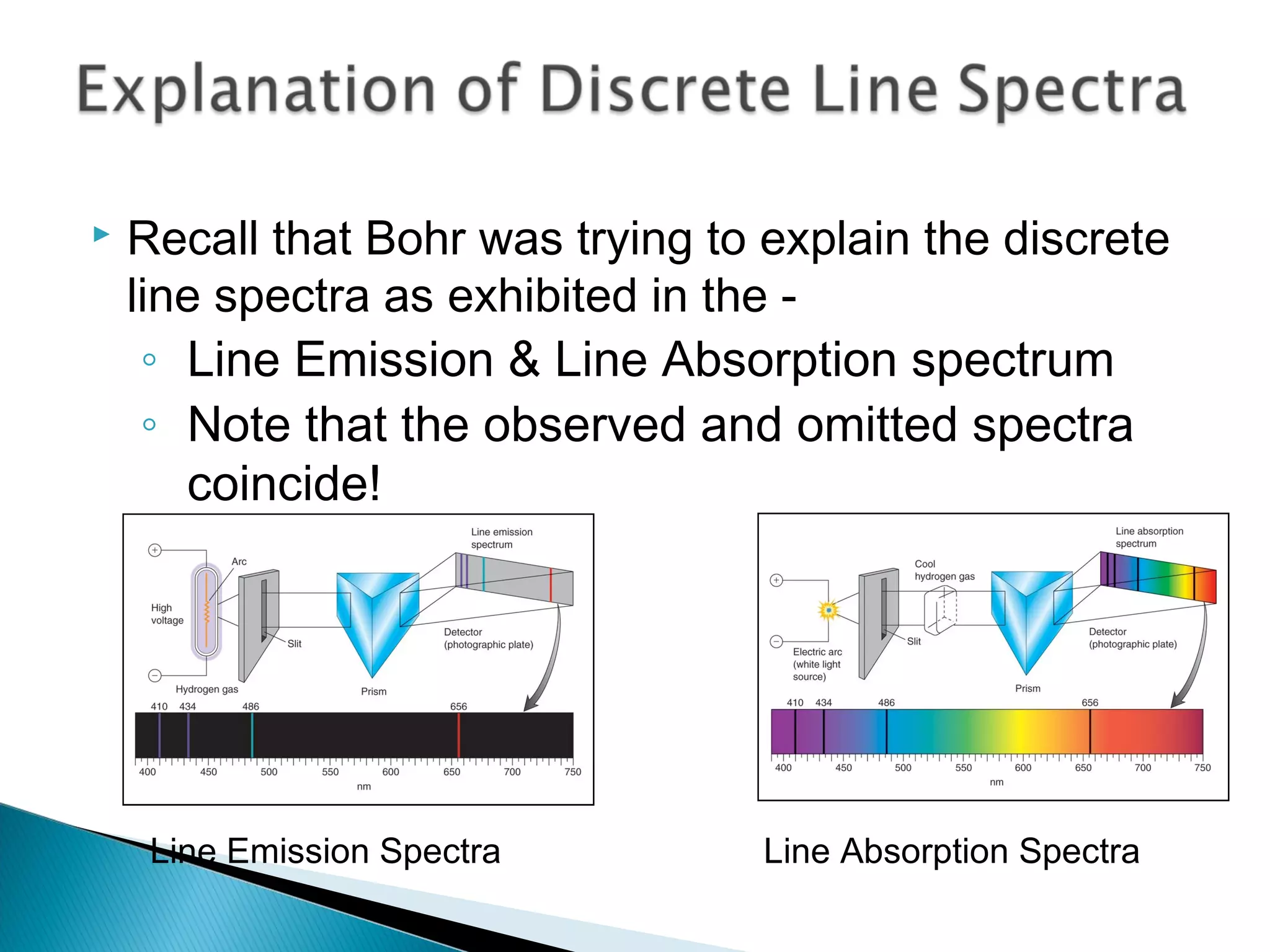
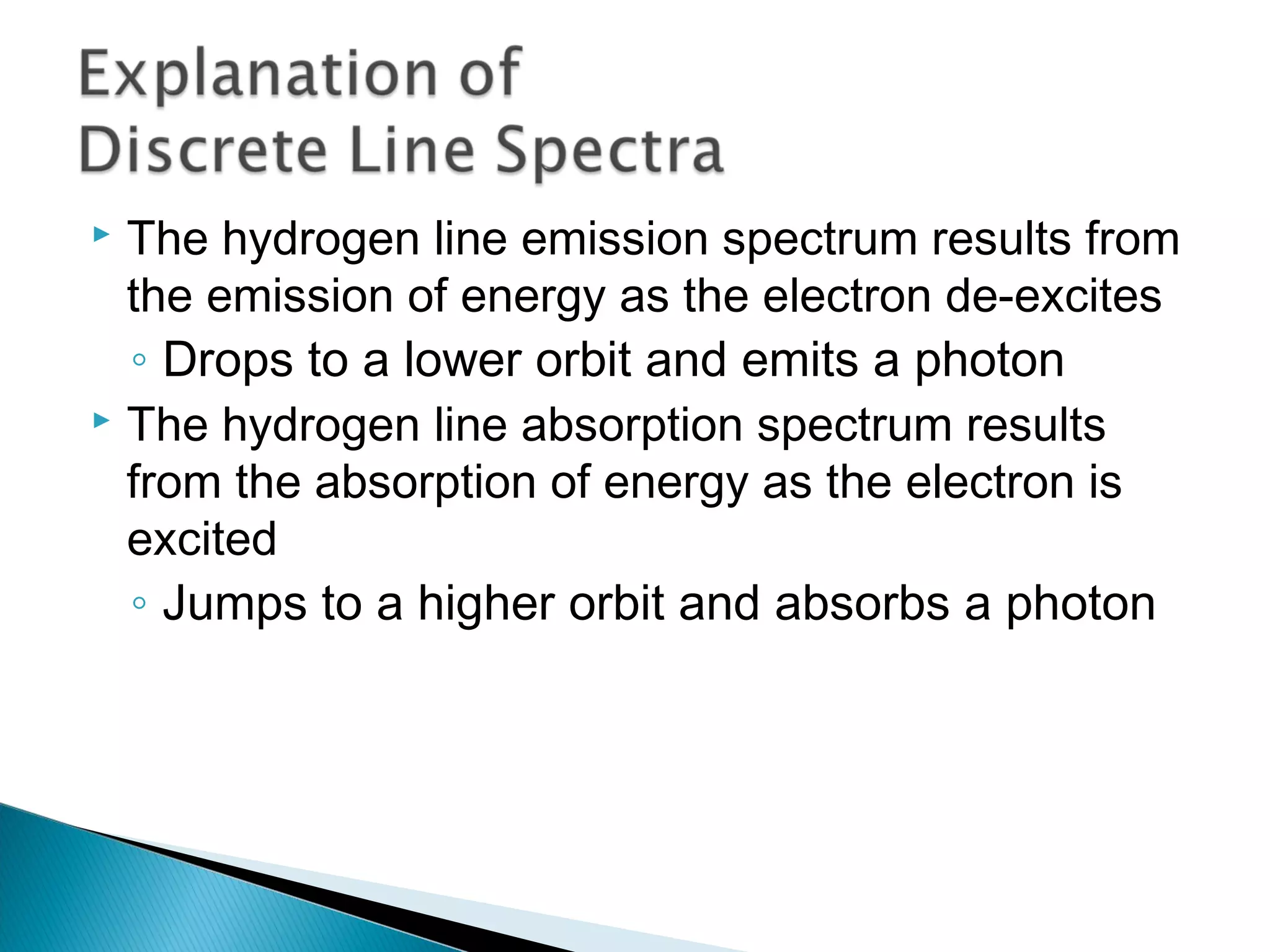
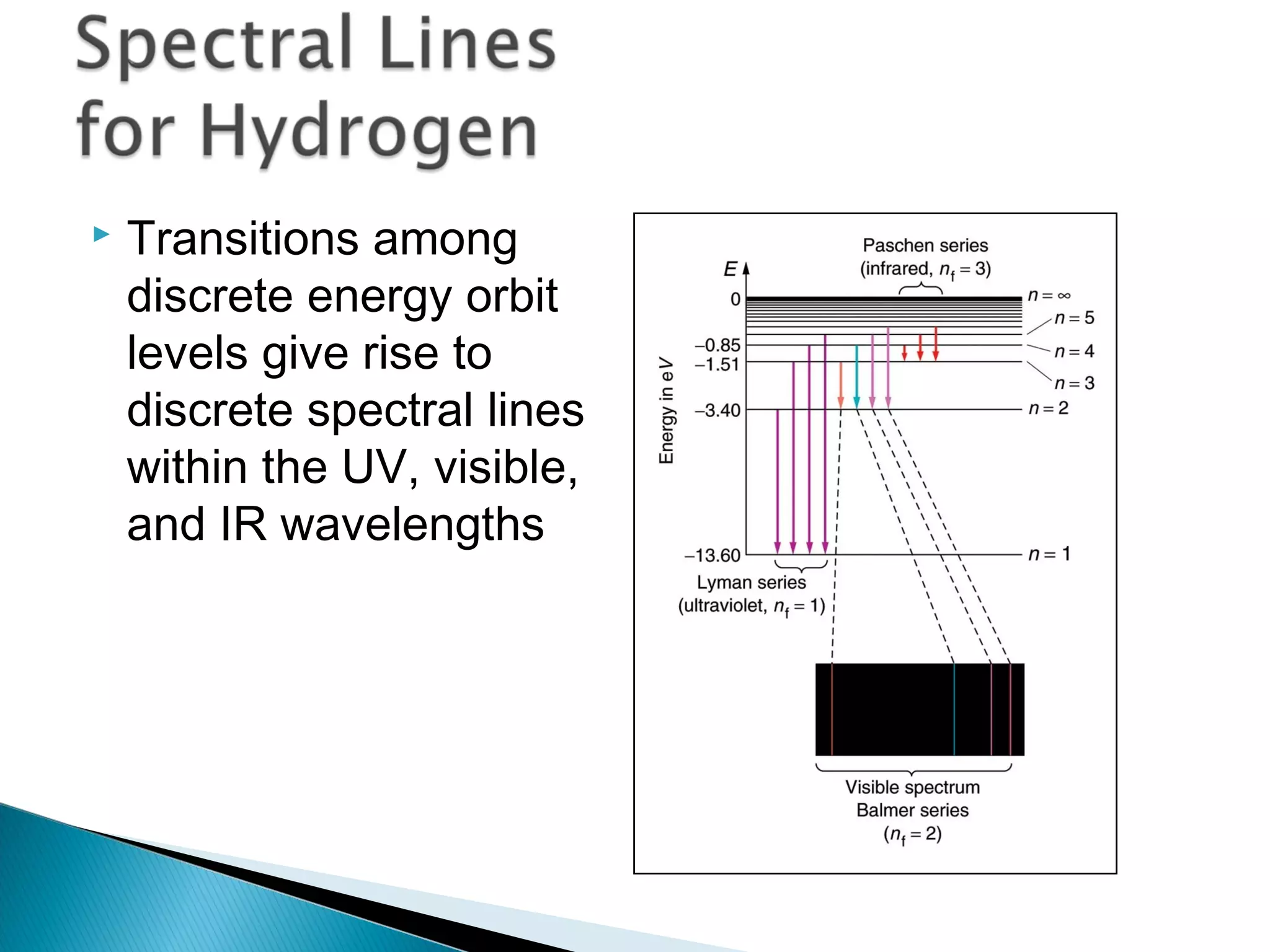
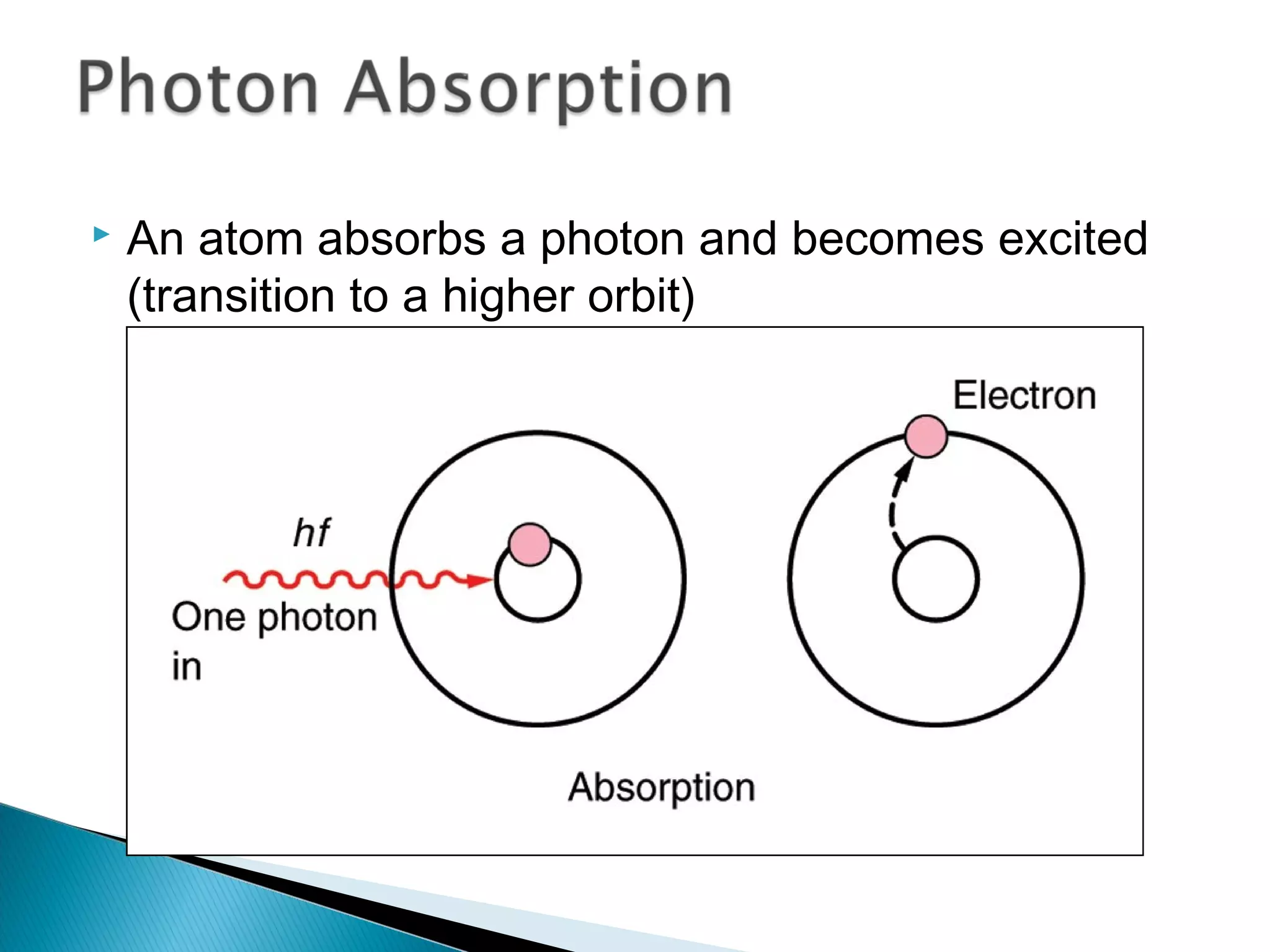
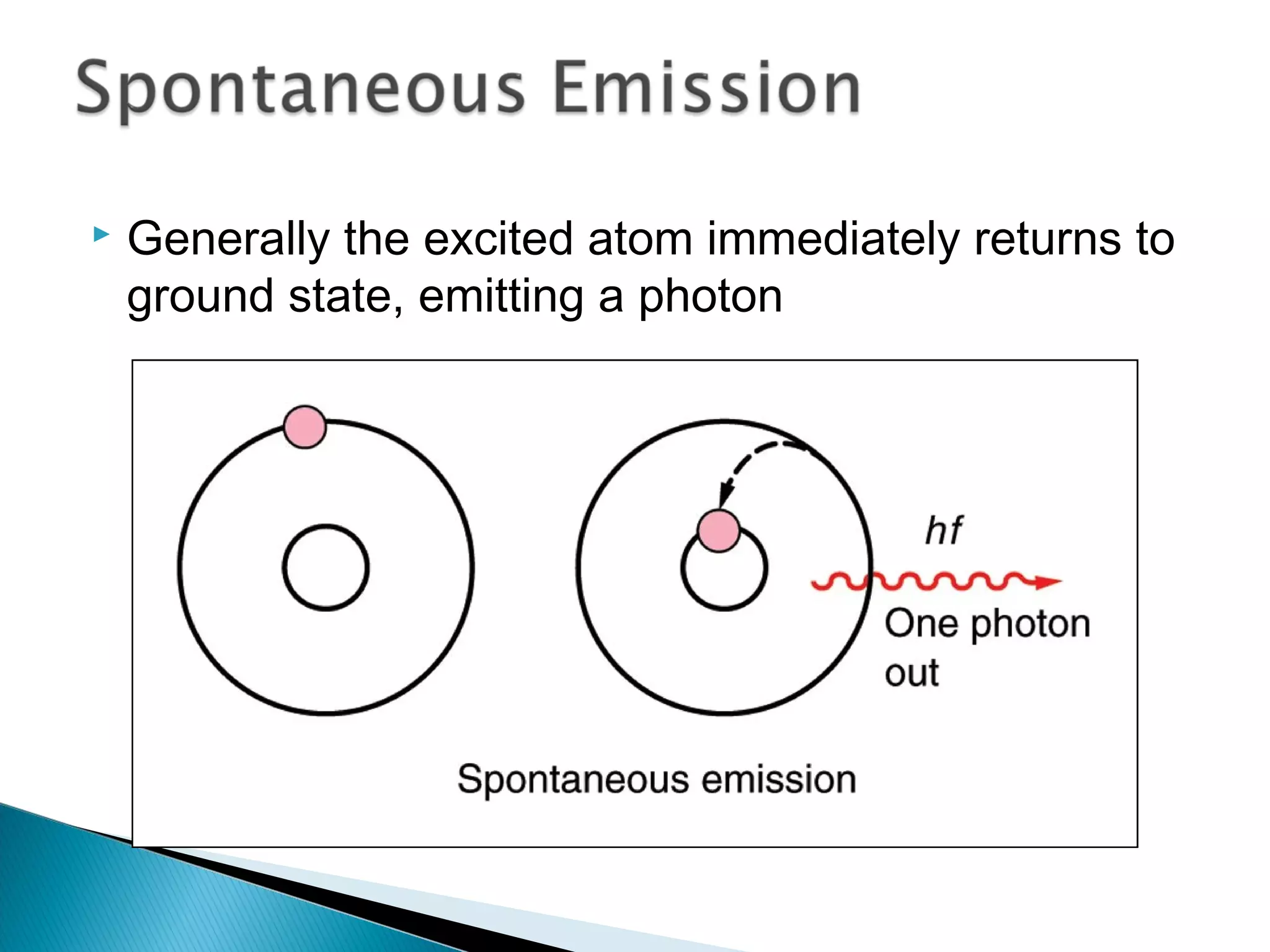
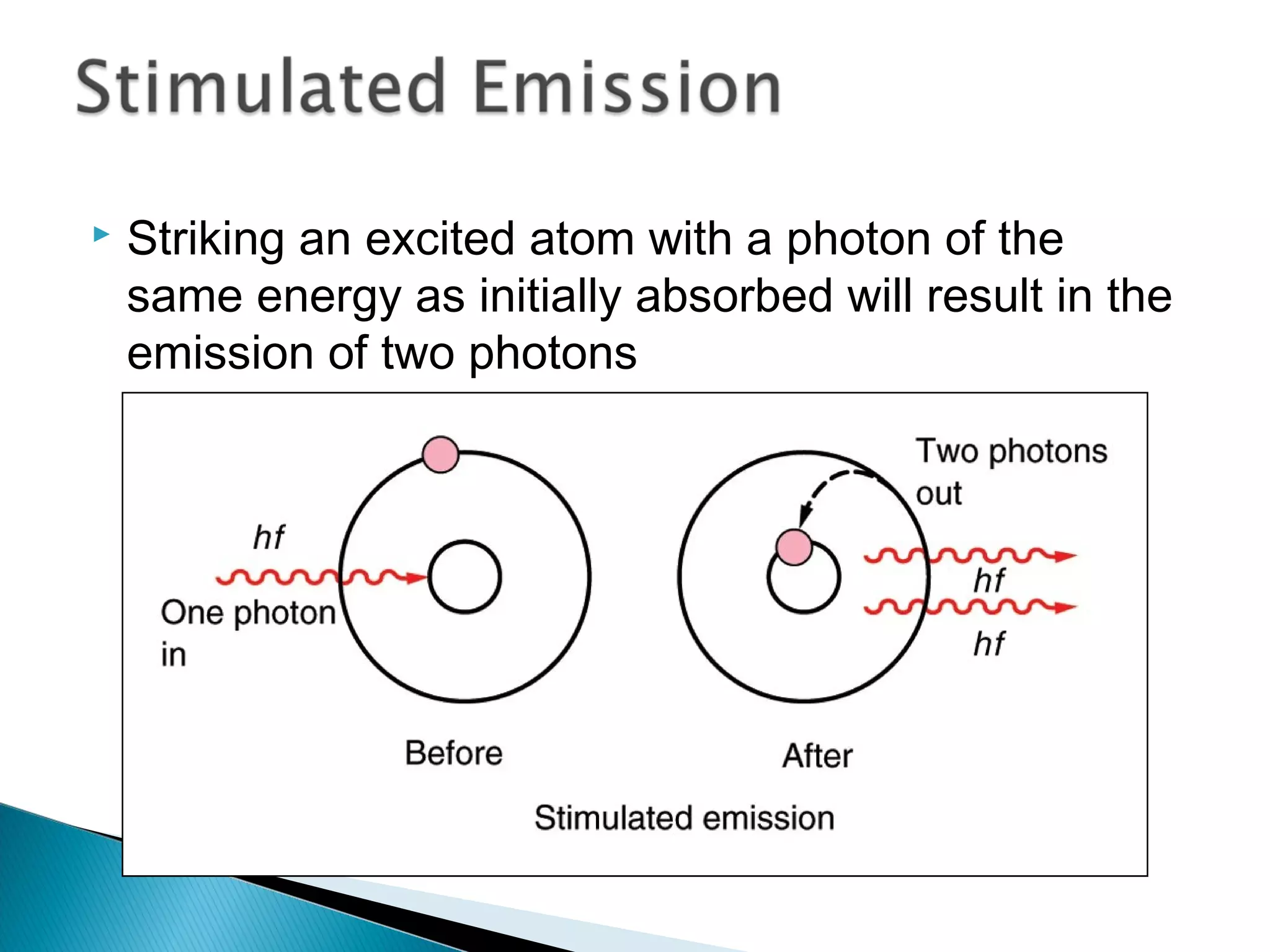
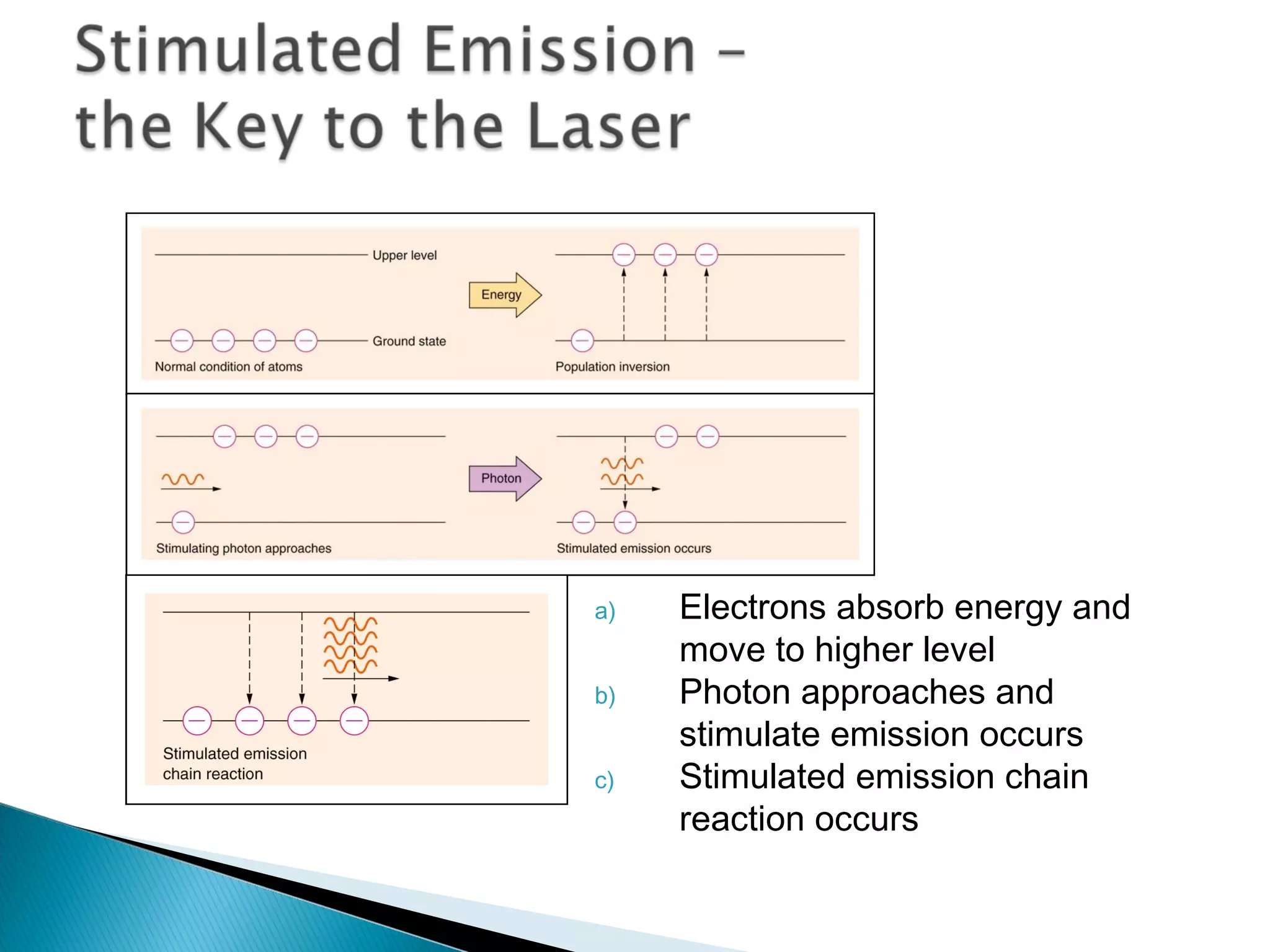
3) Bohr used Planck's idea of quantized energy to explain atomic spectra, proposing that electrons in atoms can only occupy discrete energy levels corresponding to distinct orbital radii. When electrons jump between these levels, photons of specific frequencies are emitted or absorbed.