1. Atomic structure consists of electrons, protons, and neutrons. Rutherford discovered the nucleus through alpha particle scattering experiments. Bohr proposed electrons orbit the nucleus in discrete energy levels.
2. Millikan's oil drop experiment precisely measured the charge of an electron. J.J. Thomson used cathode ray tubes to discover electrons and determine their small mass.
3. Bohr's model of the hydrogen atom explained its stable orbitals and spectral lines by postulating discrete electron energy levels and angular momentum quantization. This resolved issues with Rutherford's model of unstable orbits.
















![18
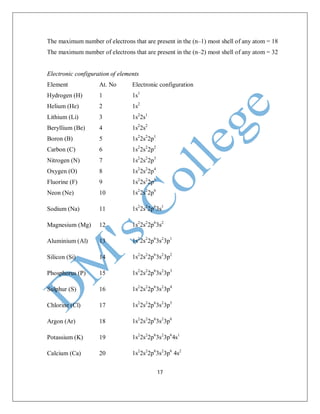
Scandium (Sc) 21 1s2
2s2
2p6
3s2
3p6
4s2
3d1
Titanium (Ti) 22 1s2
2s2
2p6
3s2
3p6
4s2
3d2
Vanadium (V) 23 1s2
2s2
2p6
3s2
3p6
4s2
3d3
Chromium (Cr) 24 1s2
2s2
2p6
3s2
3p6
4s1
3d5
Manganese (Mn) 25 1s2
2s2
2p6
3s2
3p6
4s2
3d5
Iron (Fe) 26 1s2
2s2
2p6
3s2
3p6
4s2
3d6
Cobalt (Co) 27 1s2
2s2
2p6
3s2
3p6
4s2
3d7
Nickel (Ni) 28 1s2
2s2
2p6
3s2
3p6
4s2
3d8
Copper (Cu) 29 1s2
2s2
2p6
3s2
3p6
4s1
3d10
Zinc (Zn) 30 1s2
2s2
2p6
3s2
3p6
4s2
3d10
16. Anomalous electronic configurations
Half filled and completely filled degenerate orbitals give greater stability to atoms. Cr (Z =
24) and Cu (Z = 29) have anomalous electronic configuration due to this reason. Electronic
configuration of Cr atom is 1s2
2s2
2p6
3s2
3p6
3d5
4s1
or [Ar] 3d5
4s1
but not 1s2
2s2
2p6
3s2
3p6
3d4
4s2
.
Electronic configuration of Cu atom is 1s2
2s2
2p6
3s2
3p6
3d10
4s1
or [Ar] 4s1
3d10
but not
1s2
2s2
2p6
3s2
3p6
3d9
4s2
.
17. Magnetic properties
Atoms molecules, ions or any species having unpaired electrons exhibit paramagnetism.
These are attracted into the magnetic field when they are placed in an external magnetic field.
Atoms having the completely paired electrons are repelled by the external magnetic field and are
called diamagnetic.
18. Stability of atoms](https://image.slidesharecdn.com/atomicstructure-170208042405/85/Atomic-structure-18-320.jpg)
![19
Theory of exchange forces will explain why Cr has [Ar] 3d5
4s1
but not [Ar] 3d4
4s2
.
According to this theory, greater the number of unpaired electrons, greater is the number of
possible exchange pairs of electrons and more is the exchange energy released and the atom is
more stable. For Cr → [Ar] 3d5
4s1
, the possible number of exchange pairs = 15. If energy
released for each exchange pair is k, the total exchange energy is 15 k. For Cr → [Ar] 3d4
4s2
,
the possible number of exchange pairs = 10 and total exchange energy is only 10k. Therefore Cr
→[Ar] 3d5
4s1
is more stable than Cr(Ar) 3d4
4s2
.
19. Effective nuclear charge concept: The Slater’s Rules
Consider an electron is one of the atomic orbitals of a multi electron atom. Because of the
electrostatic repulsion by the other electrons in the same or other orbitals, the nuclear change felt
by this electron will be less than the actual nuclear charge. This electron is said to be screened
from the influence of the nuclear charge and the reduced charge felt by the electron is known as
the effective nuclear charge.
The effective nuclear charge for any electron in the configuration of an atom may be calculated
with the help of the correlation.
Zeff = Z actual-S
where‘s’ is screening constant / shielding constant.
Screening constant is evaluated by the following empirical rules.
1. The various orbital are grouped as follows and written in the order starting from the side of
the nucleus (1S) (2s3p) (3s3p) (3d) (4s4p) (4d) (4f) (5s5p) etc.
2. For an electron in a group of s,p electrons , the value of screening constant ‘S’ is the sum of
the following contribution.
i. No contribution from any electron present in the groups of orbitals lying on the right side
of the group in which the electron for which S is to be determined is present.
ii. A contribution of 0.35 from every other electron present in the group of orbitals (s,p)
under consideration. A contribution of 0.3 from the electron for which S is to be
calculated belongs to 1s orbital.
iii. A contribution of 0.85 per electron from all electrons with quantum number (n-1)](https://image.slidesharecdn.com/atomicstructure-170208042405/85/Atomic-structure-19-320.jpg)
