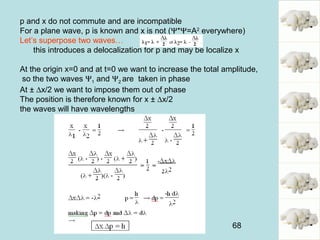
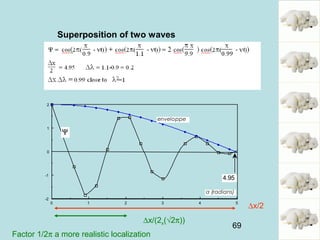
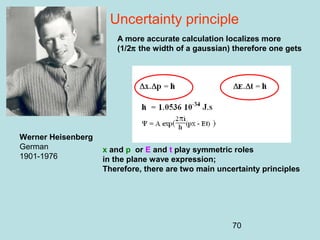
This document provides an introduction to quantum mechanics, covering several key topics:
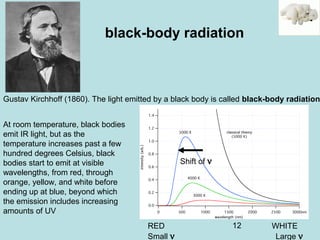
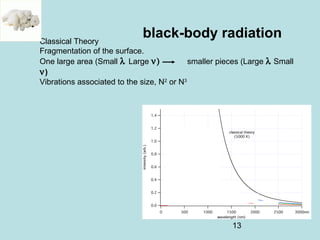
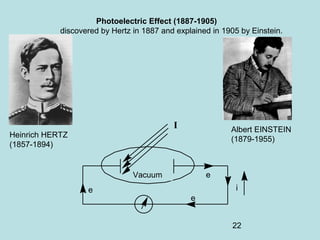
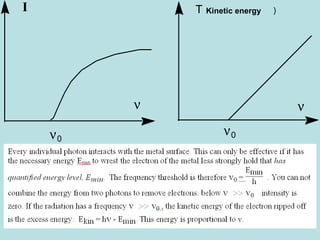
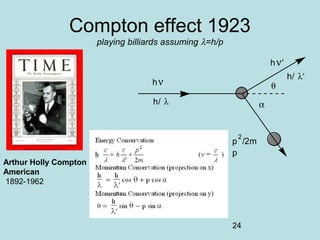
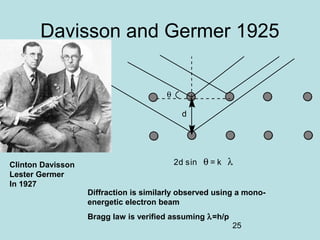
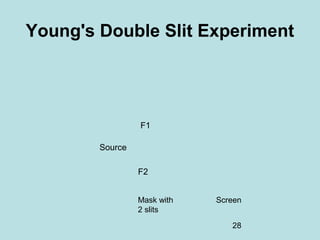
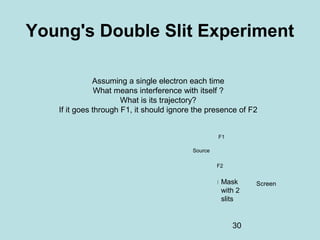
1) It discusses early discoveries that led to the development of quantum mechanics, including black-body radiation, atomic spectroscopy, the photoelectric effect, and wave-particle duality experiments like the Compton effect and electron diffraction.
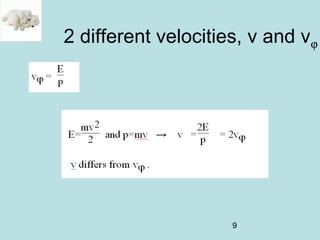
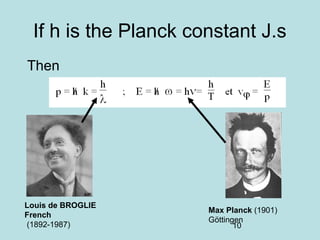
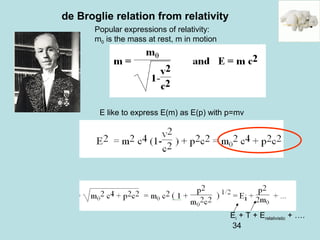
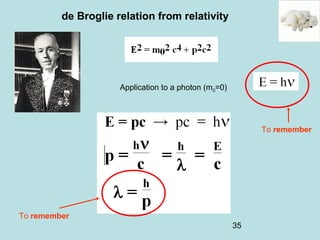
2) It introduces Louis de Broglie's hypothesis that particles are also waves, with wavelength related to momentum. This helped establish the wave-particle duality central to quantum mechanics.
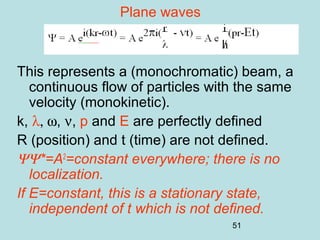
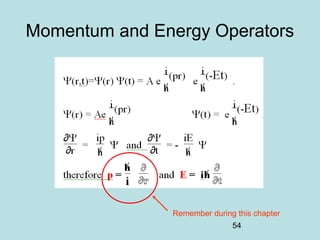
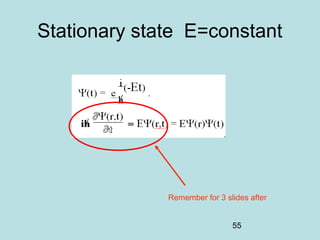
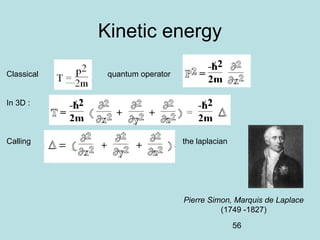
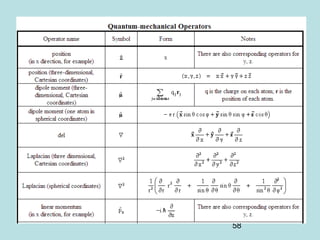
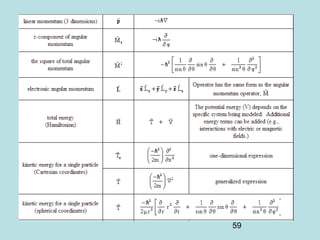
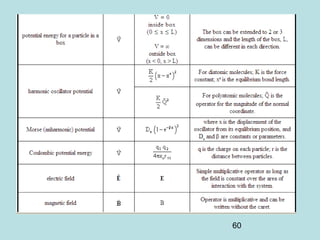
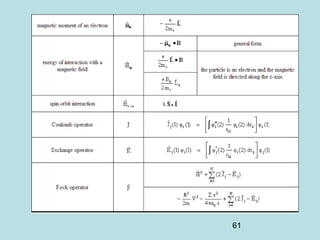
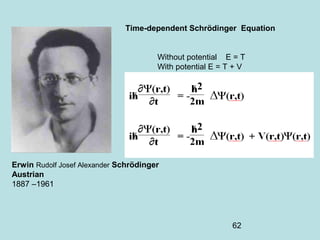
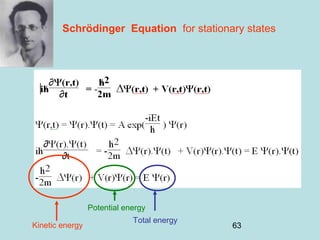
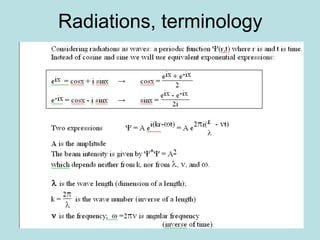
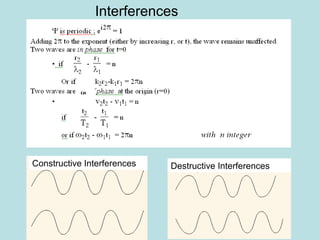
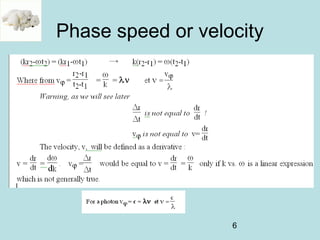
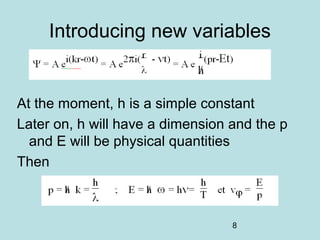
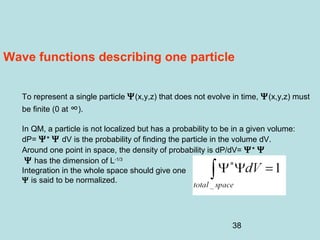
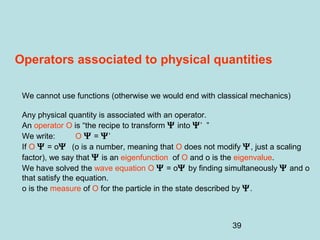
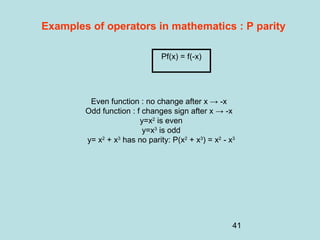
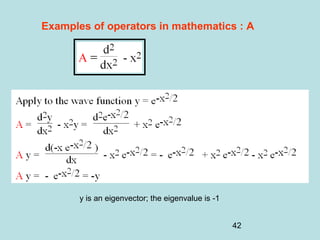
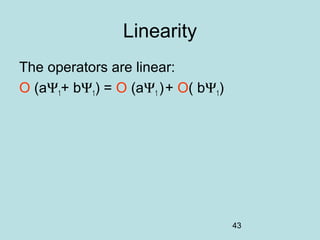
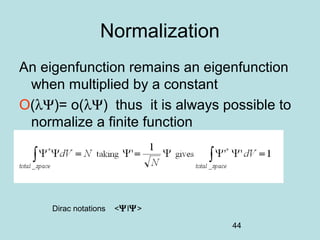
3) It describes the development of new mathematical tools in quantum mechanics, including using wave functions and operators to represent physical quantities and their associated measurements. The Schrödinger equation allows determining the wave function and eigenvalues of









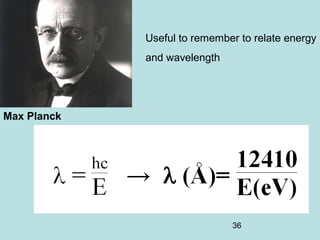


![47
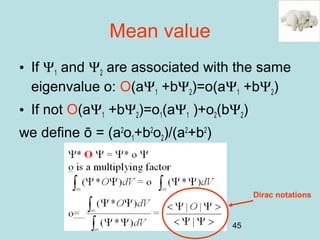
Sum, product and commutation of operators
y1=e4x
y2=x2
y3=1/x
A = d/dx 4 -- --
B = x3 3 3 3
C= x d/dx -- 2 -1
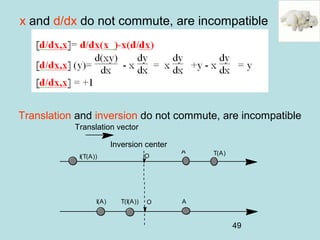
not compatible
operators
[A,C]=AC-CA≠0
[A,B]=AB-BA=0
[B,C]=BC-CB=0](https://image.slidesharecdn.com/introduction2qm-190615062905/85/Introduction2-qm-47-320.jpg)
![48
Compatibility, incompatibility of operators
y1=e4x
y2=x2
y3=1/x
A = d/dx 4 -- --
B = x3 3 3 3
C= x d/dx -- 2 -1
not compatible
operators
[A,C]=AC-CA≠0
[A,B]=AB-BA=0
[B,C]=BC-CB=0
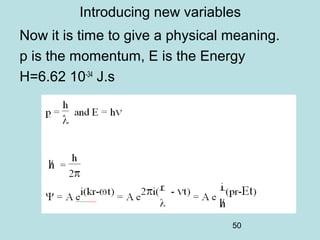
When operators commute, the physical quantities
may be simultaneously defined (compatibility)
When operators do not commute, the physical
quantities can not be simultaneously defined
(incompatibility)
compatible
operators](https://image.slidesharecdn.com/introduction2qm-190615062905/85/Introduction2-qm-48-320.jpg)