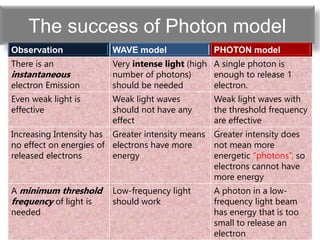
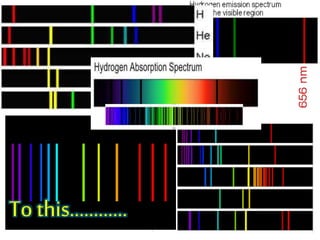
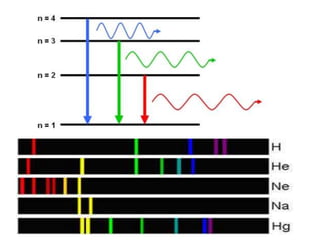
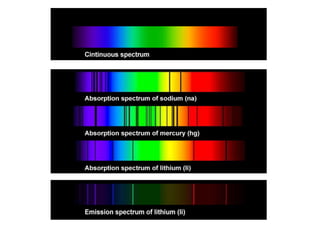
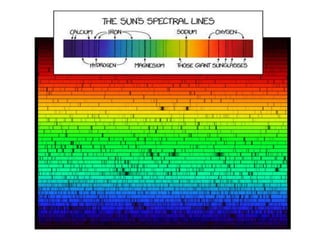
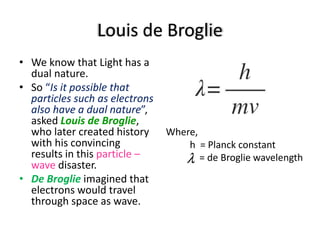
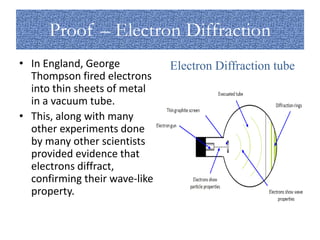
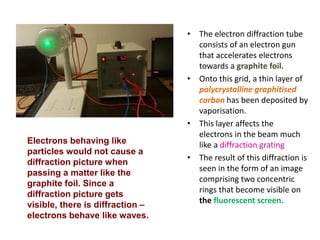
The document discusses the dual nature of light and particles, specifically photons, and the principles of wave-particle duality in physics, emphasizing phenomena such as the photoelectric effect and electron diffraction. It illustrates how light behaves as both a wave and a particle, with explanations supported by historical experiments and theories from physicists like Einstein and de Broglie. Key concepts include energy transfer through waves, the emission and absorption line spectra, and the importance of threshold frequency in photon energy release.