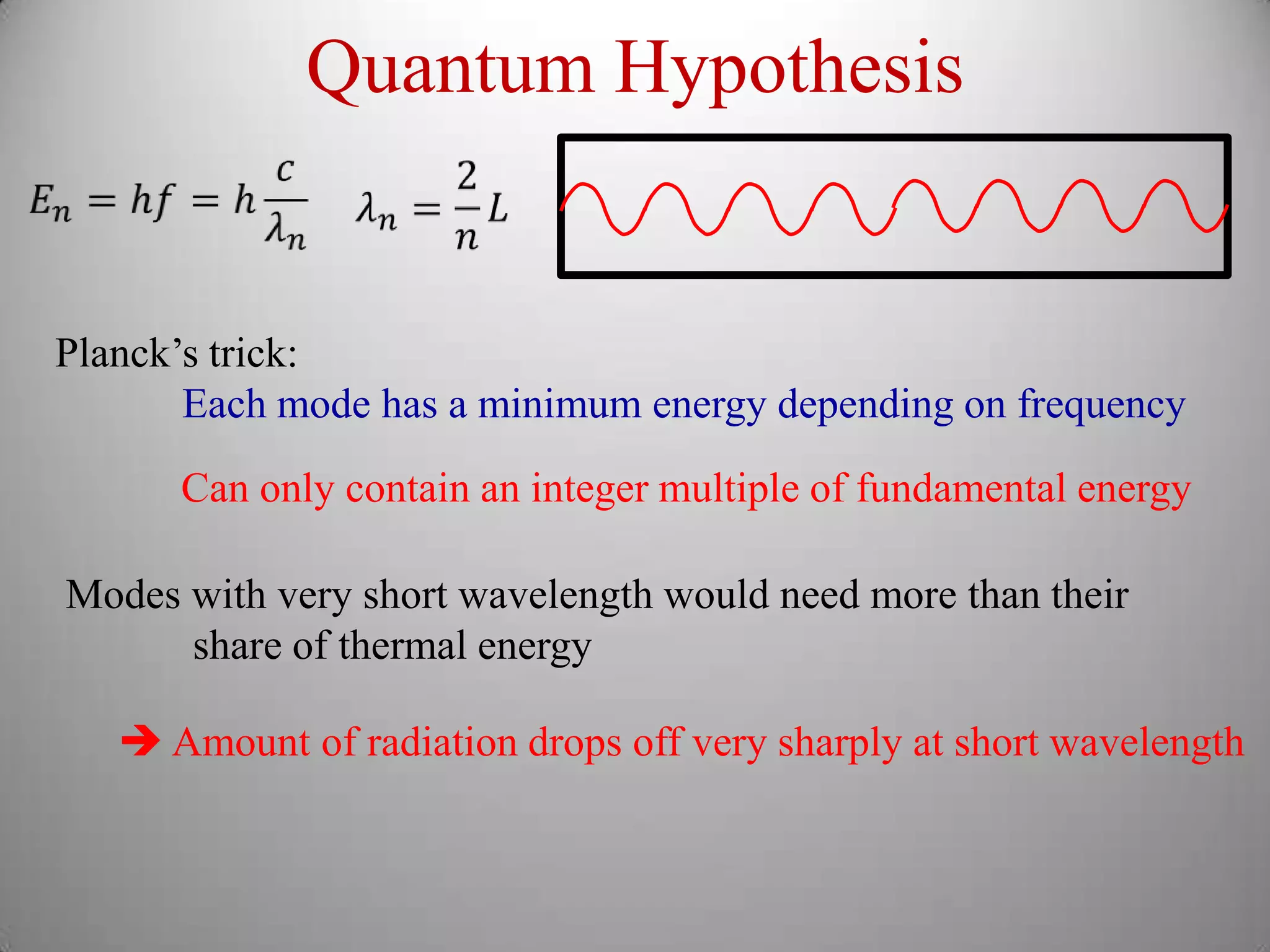
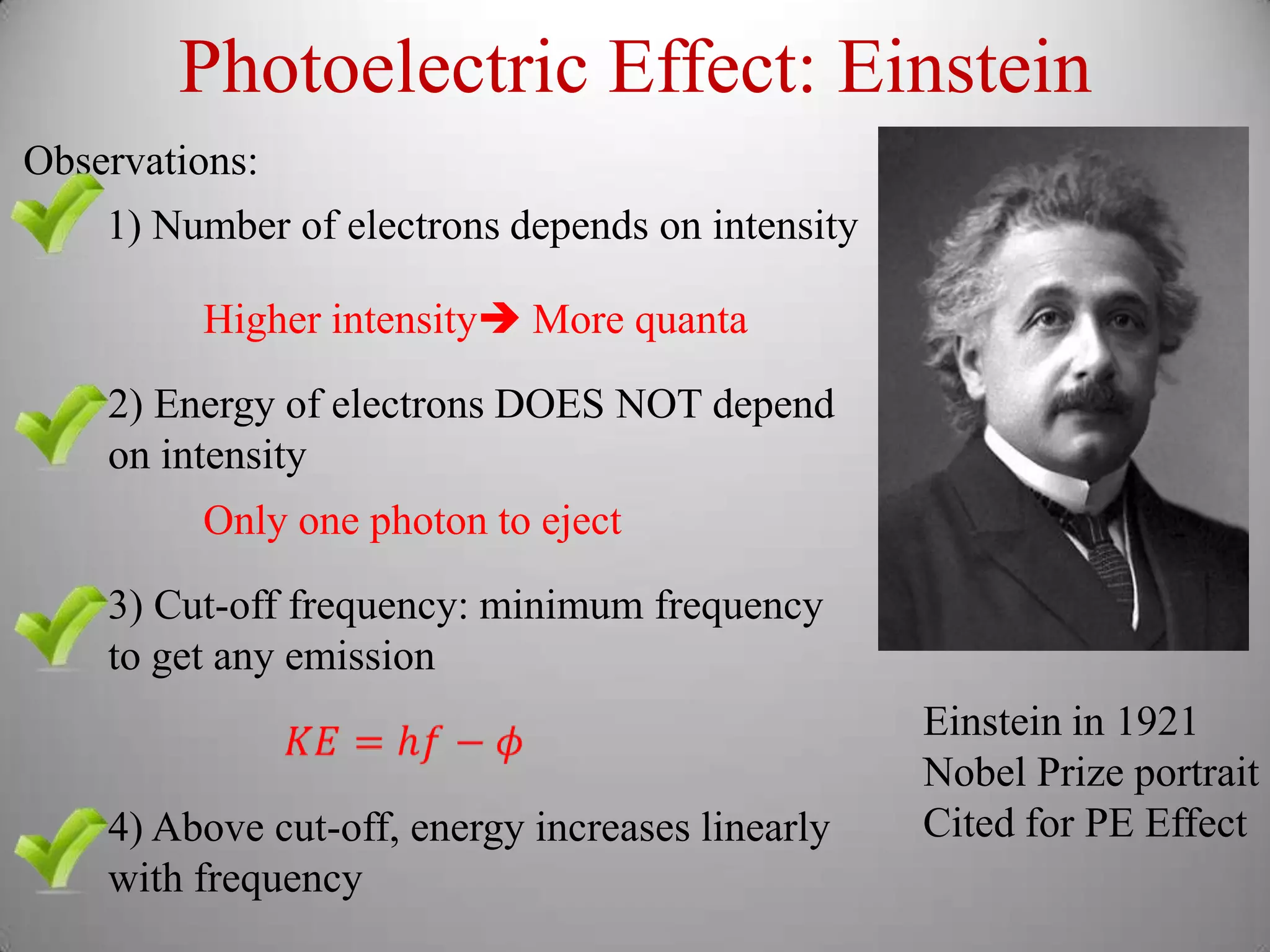
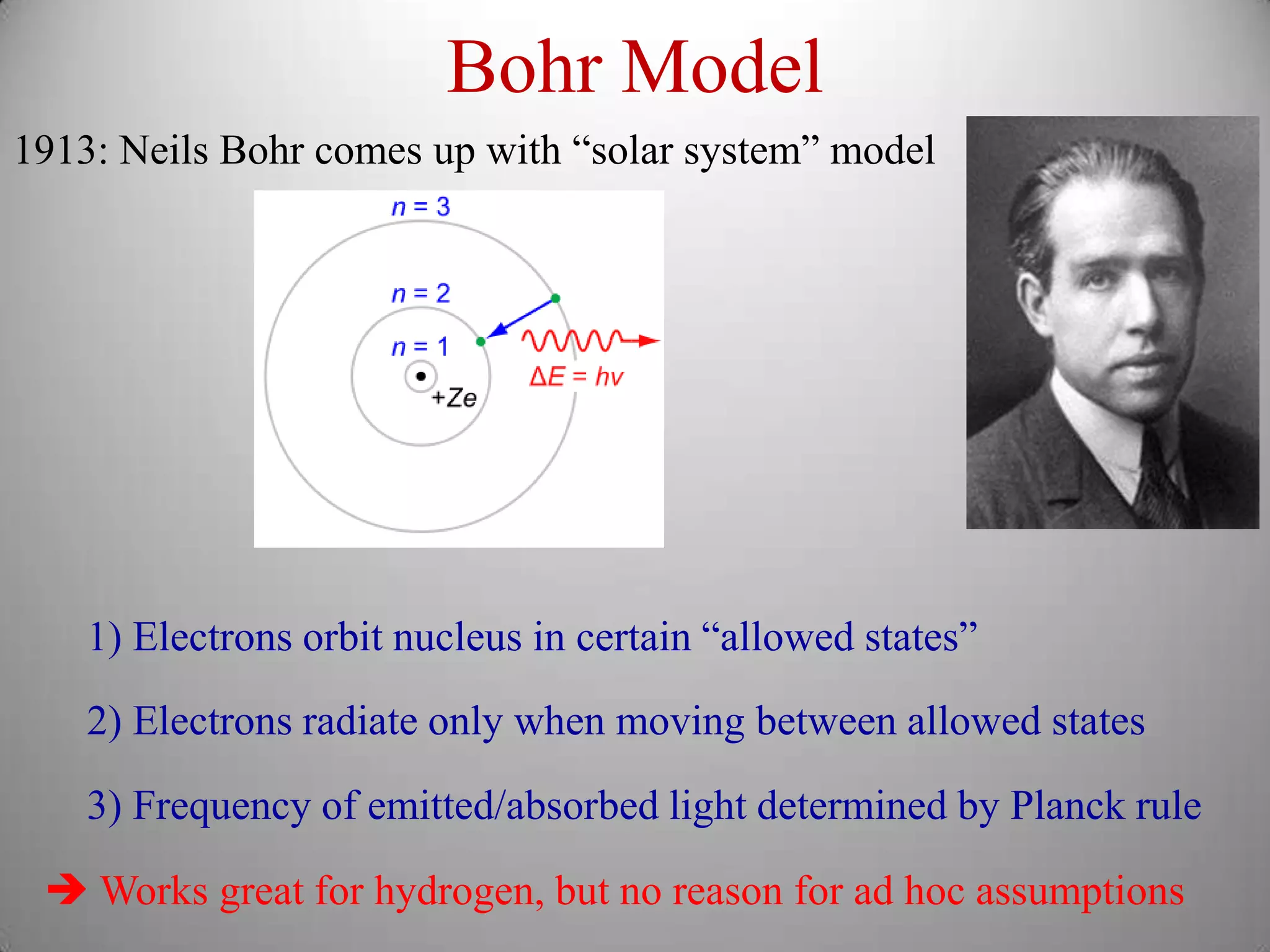
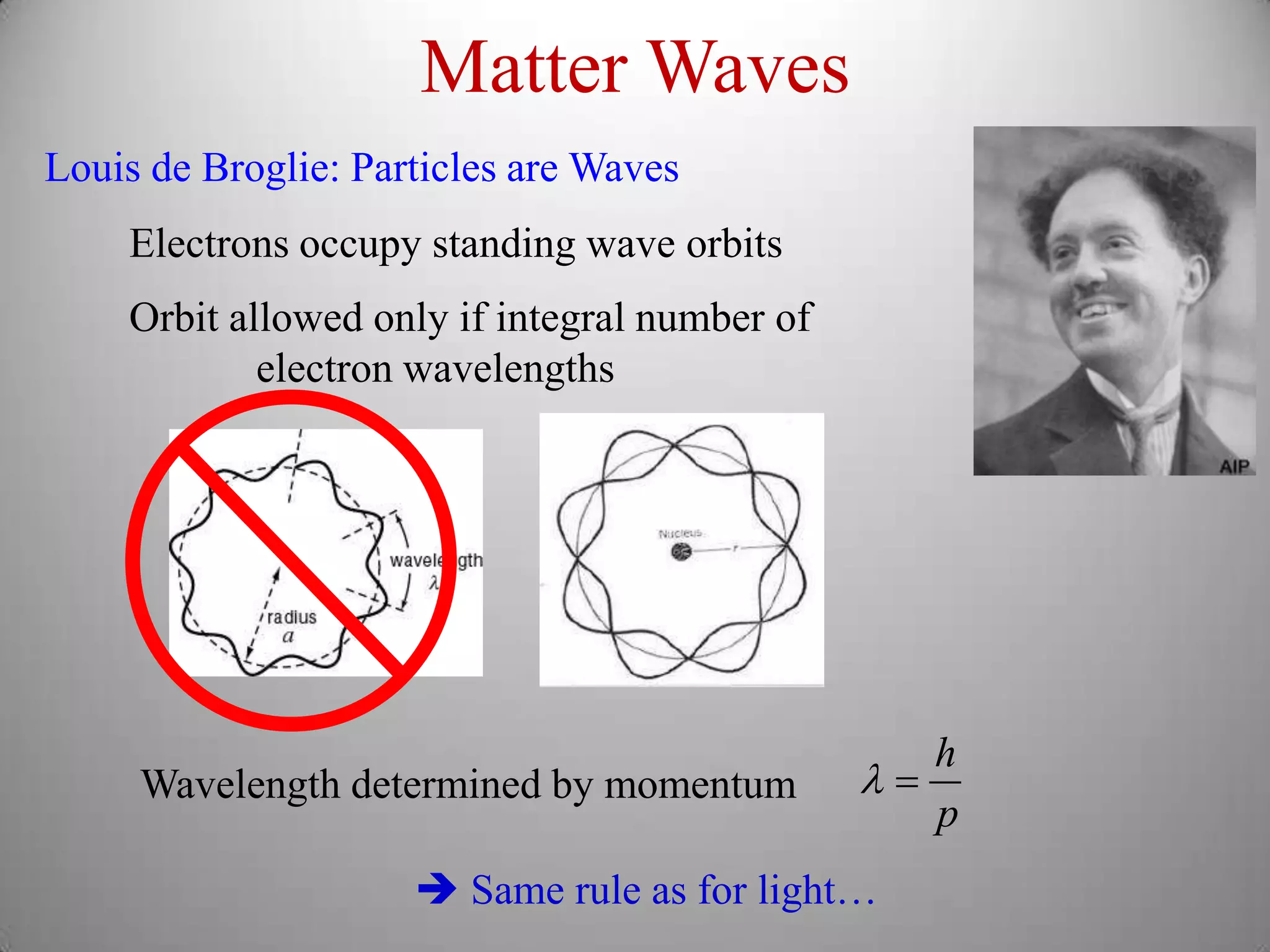
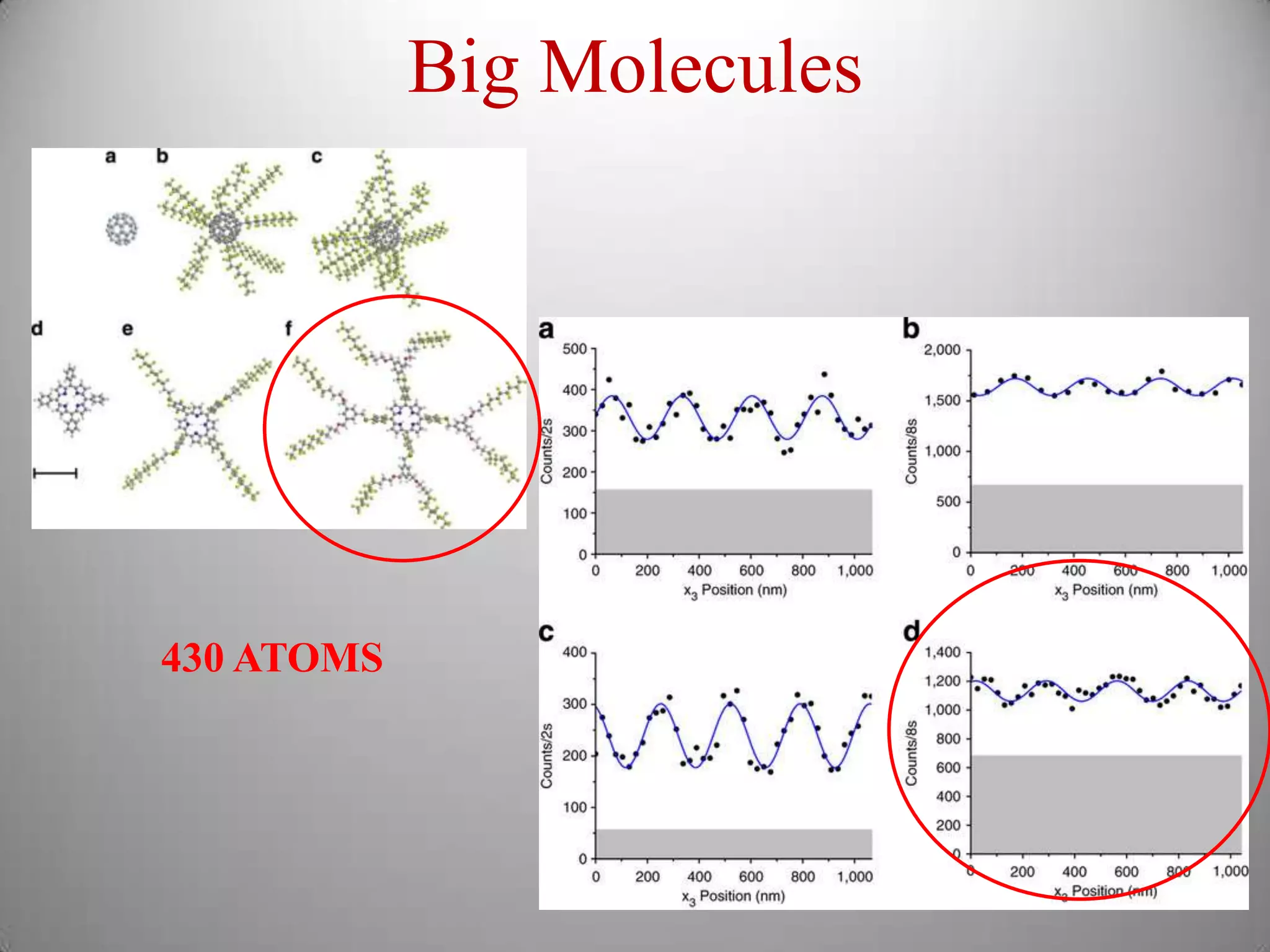
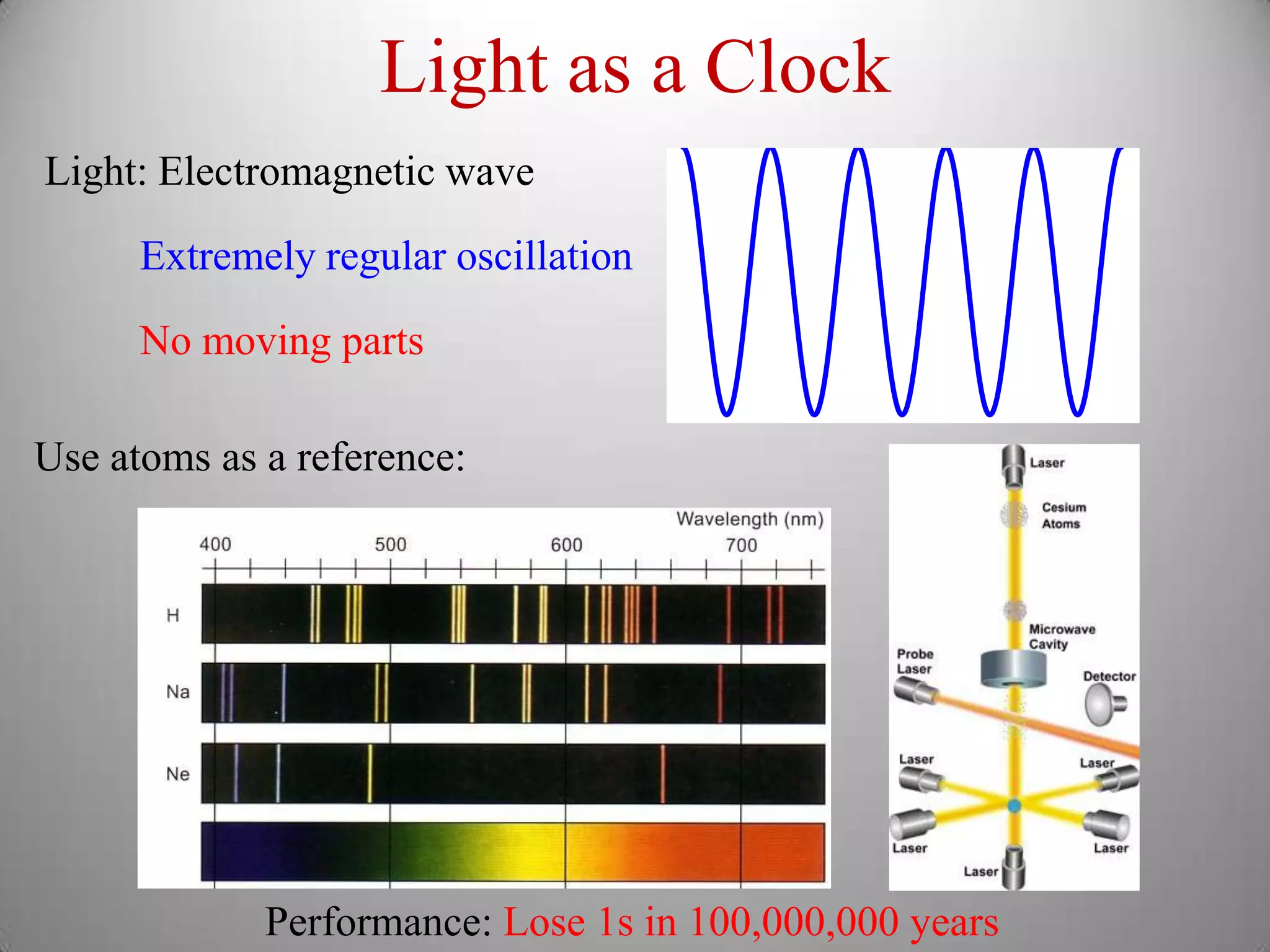
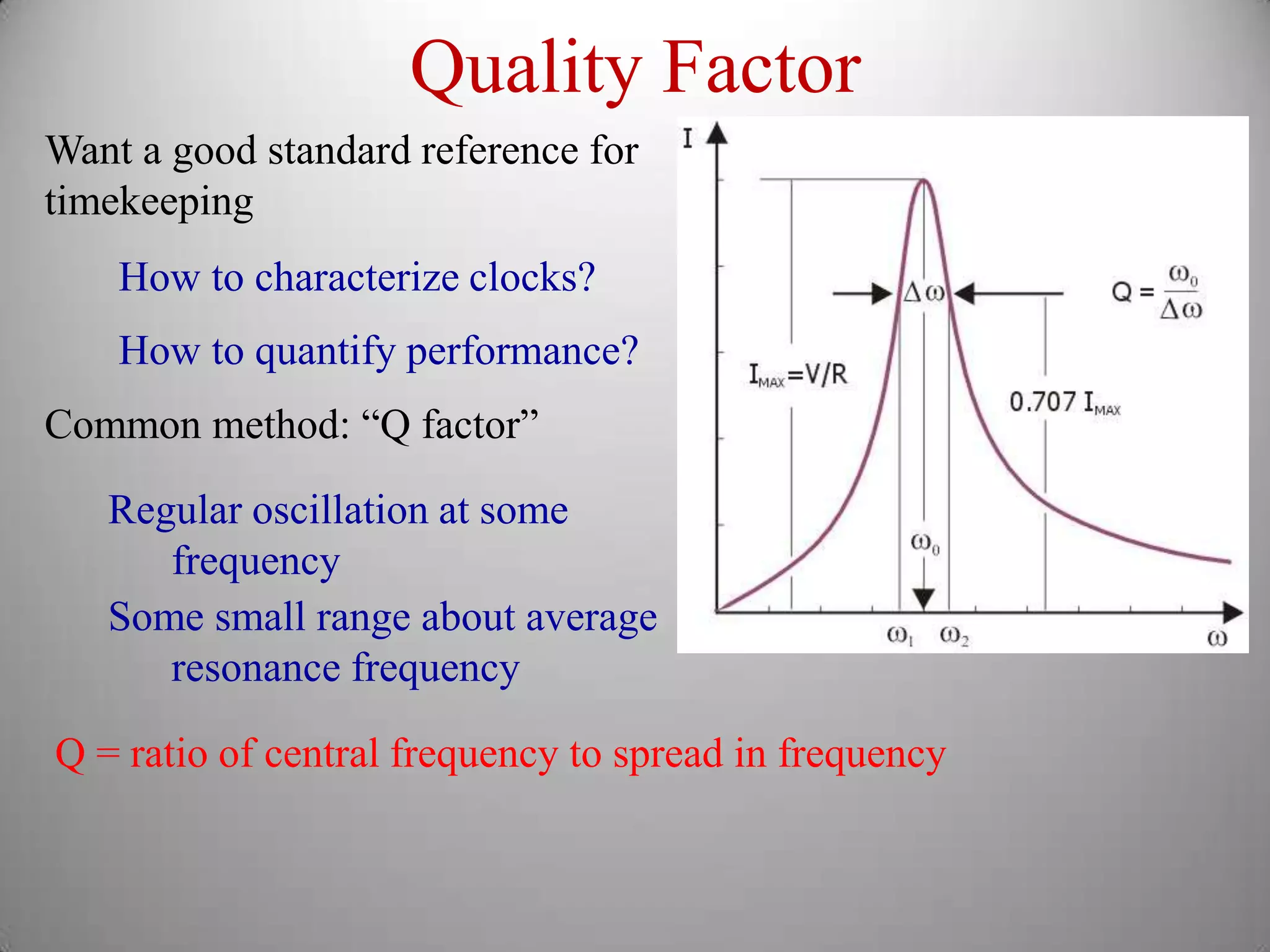
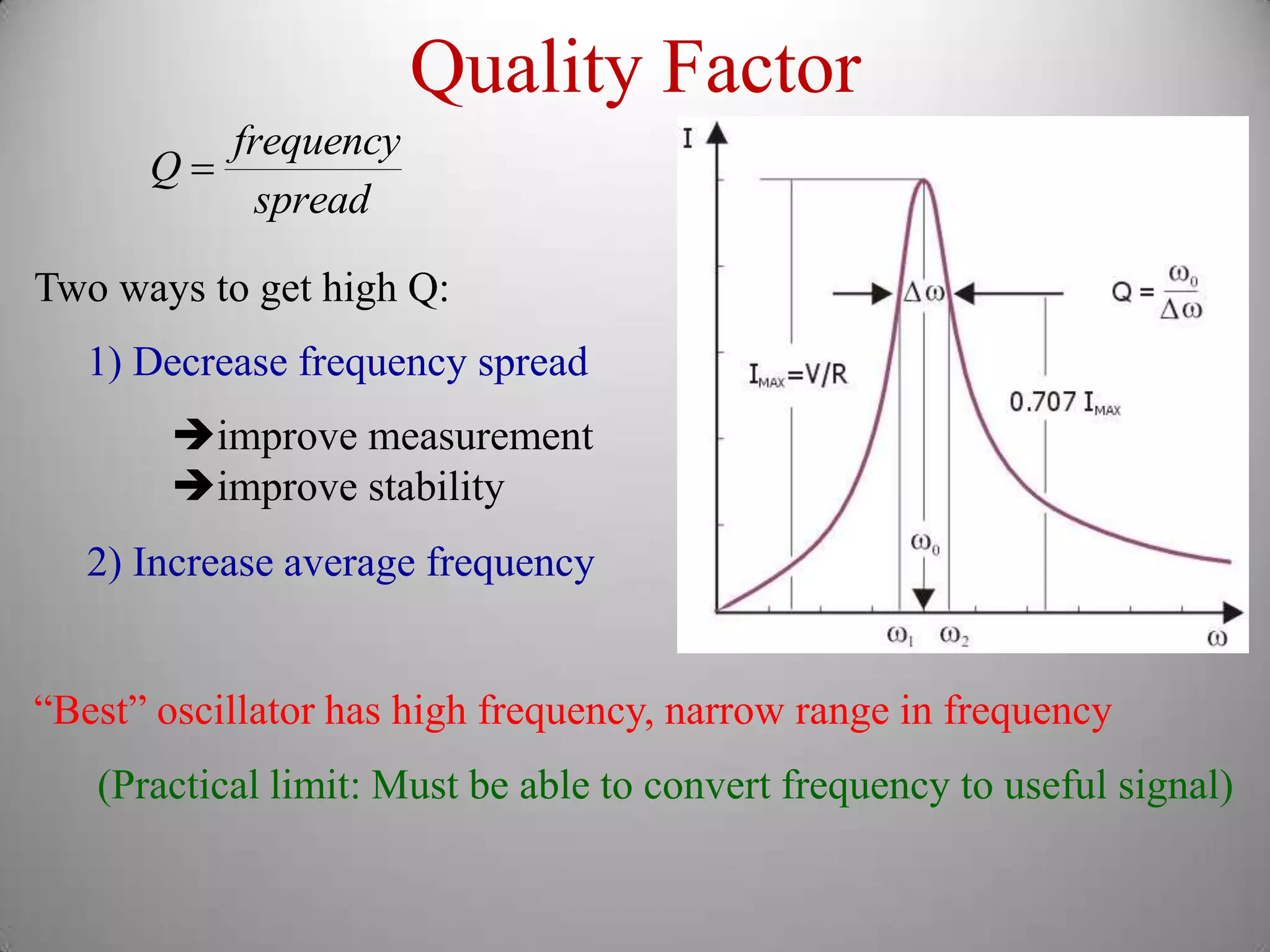
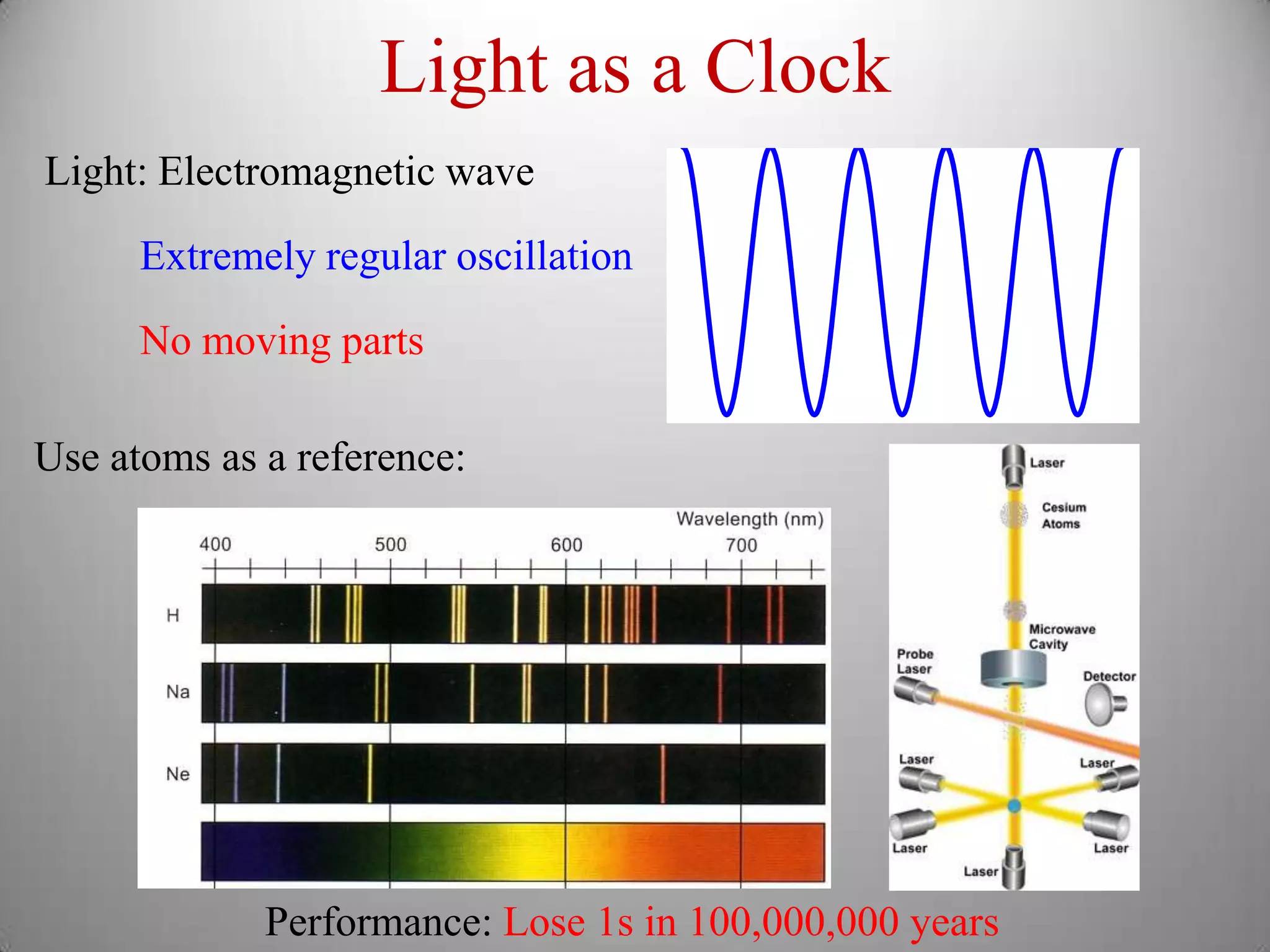
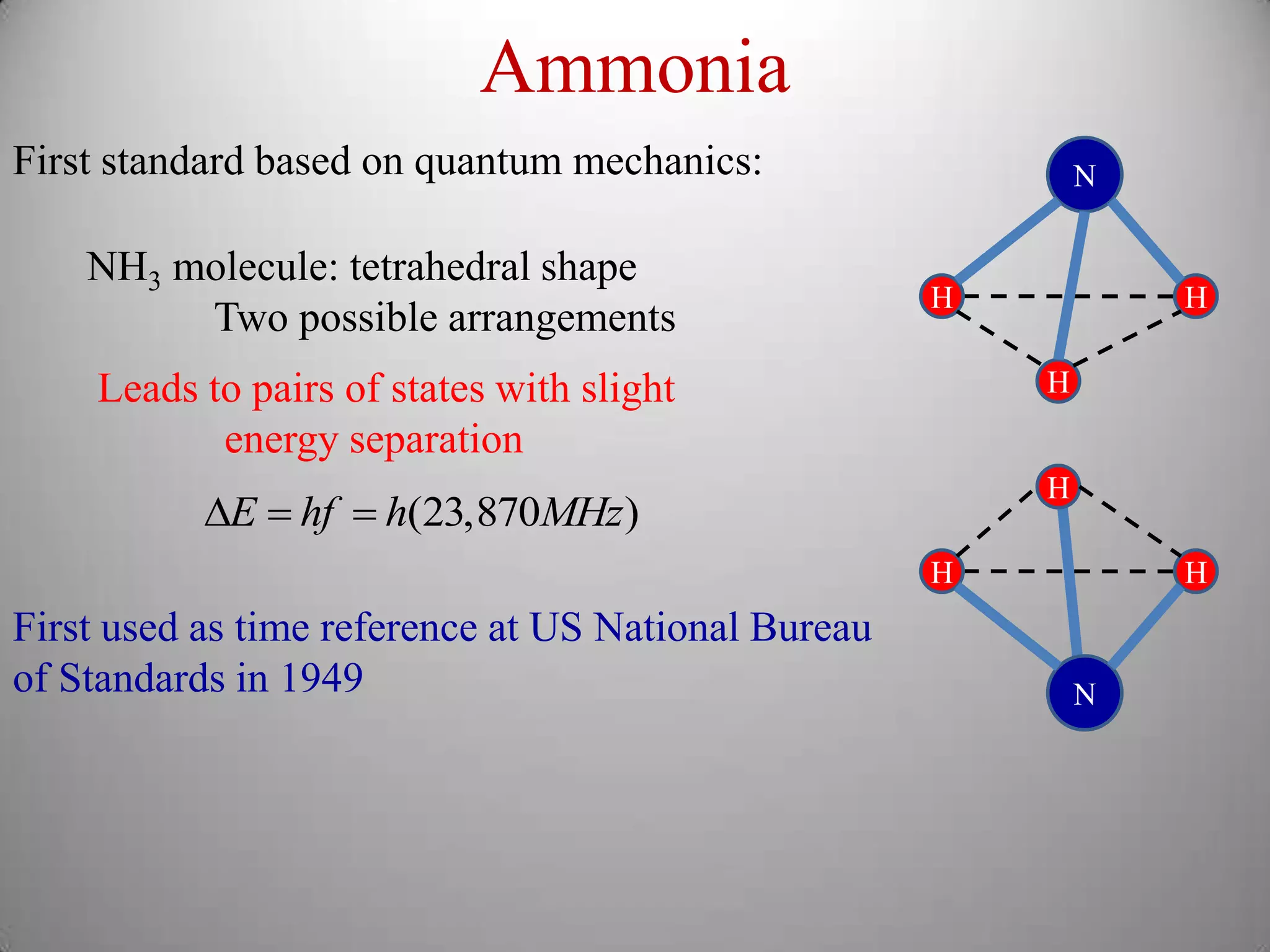
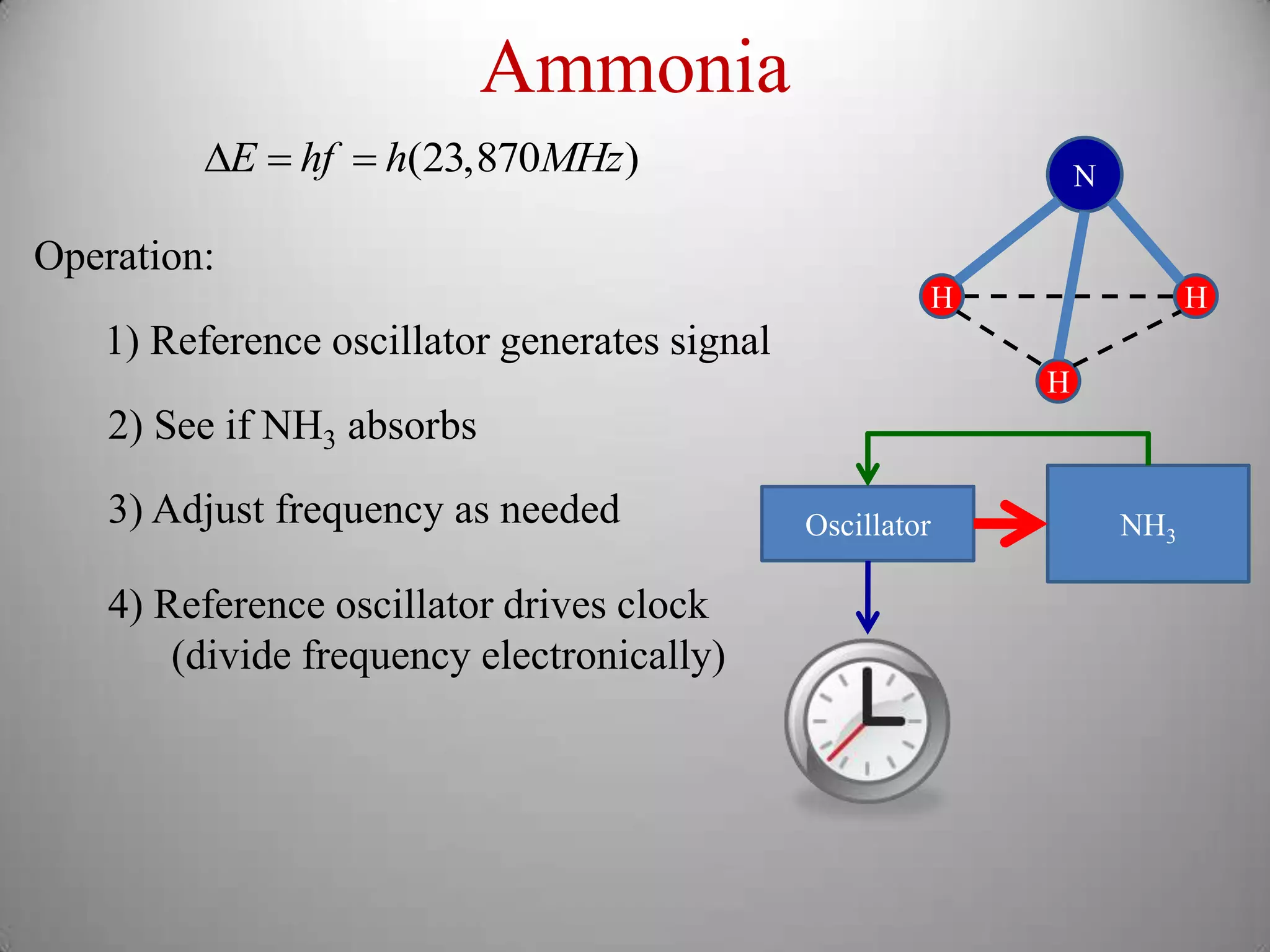
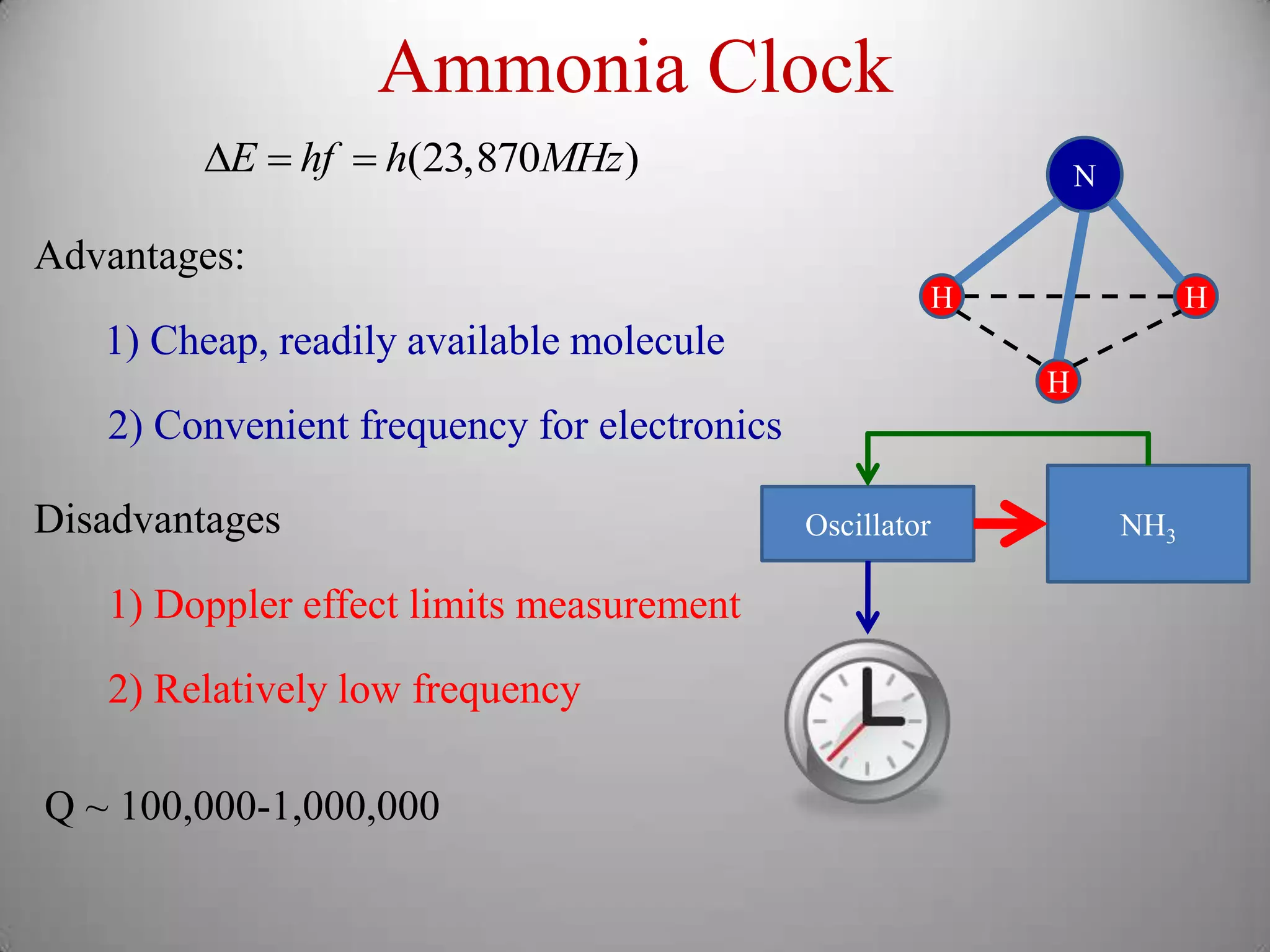
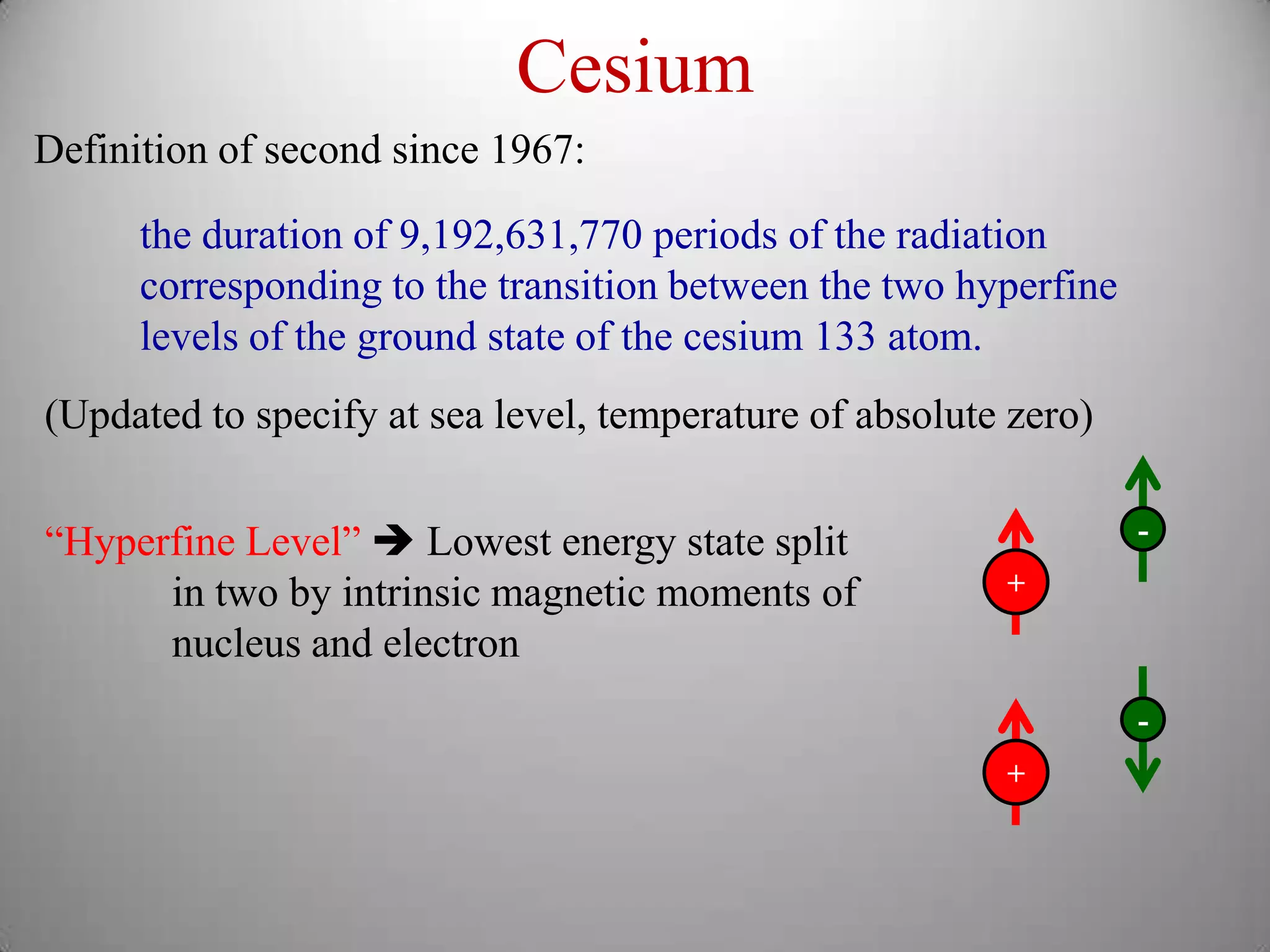
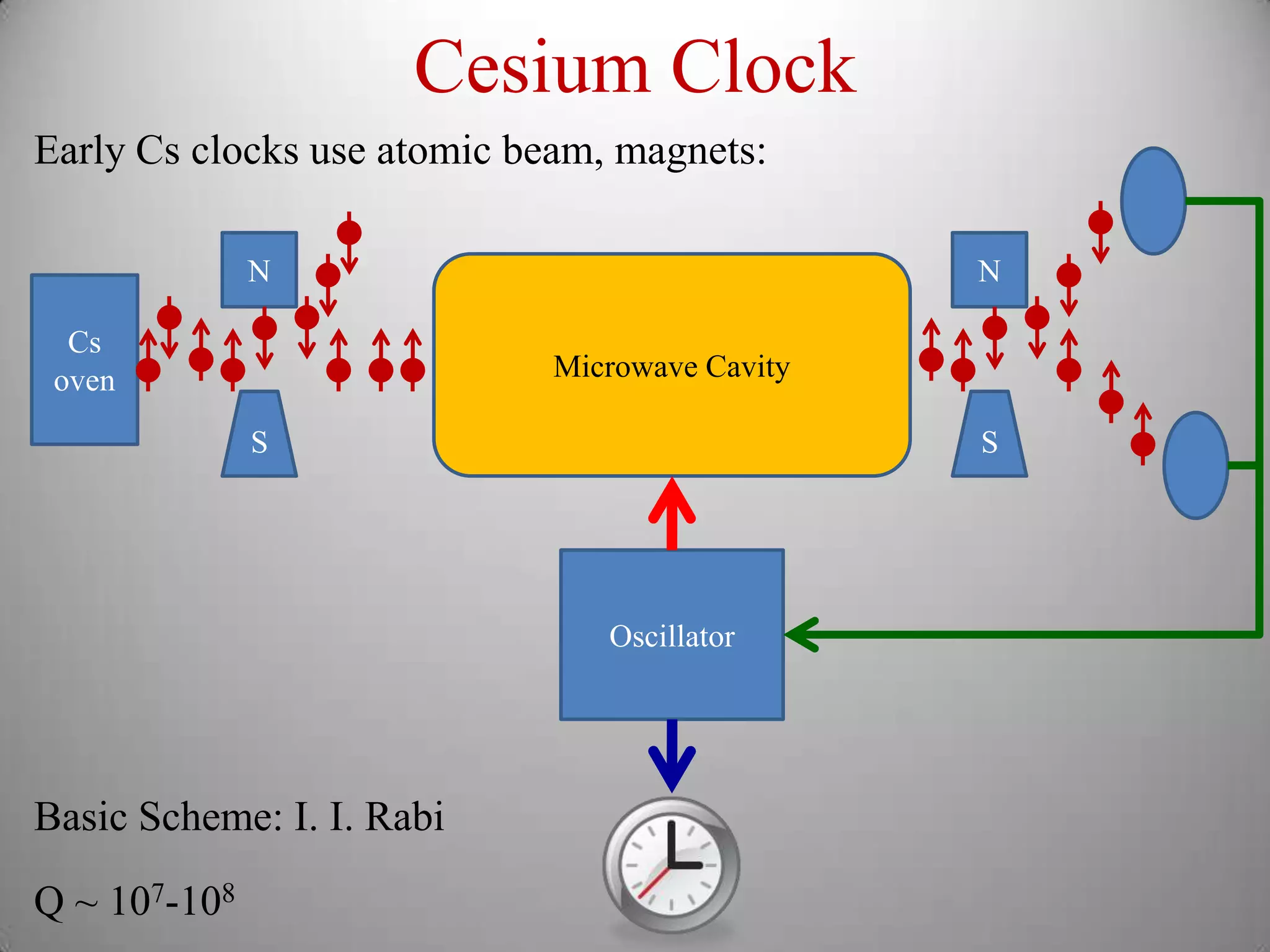
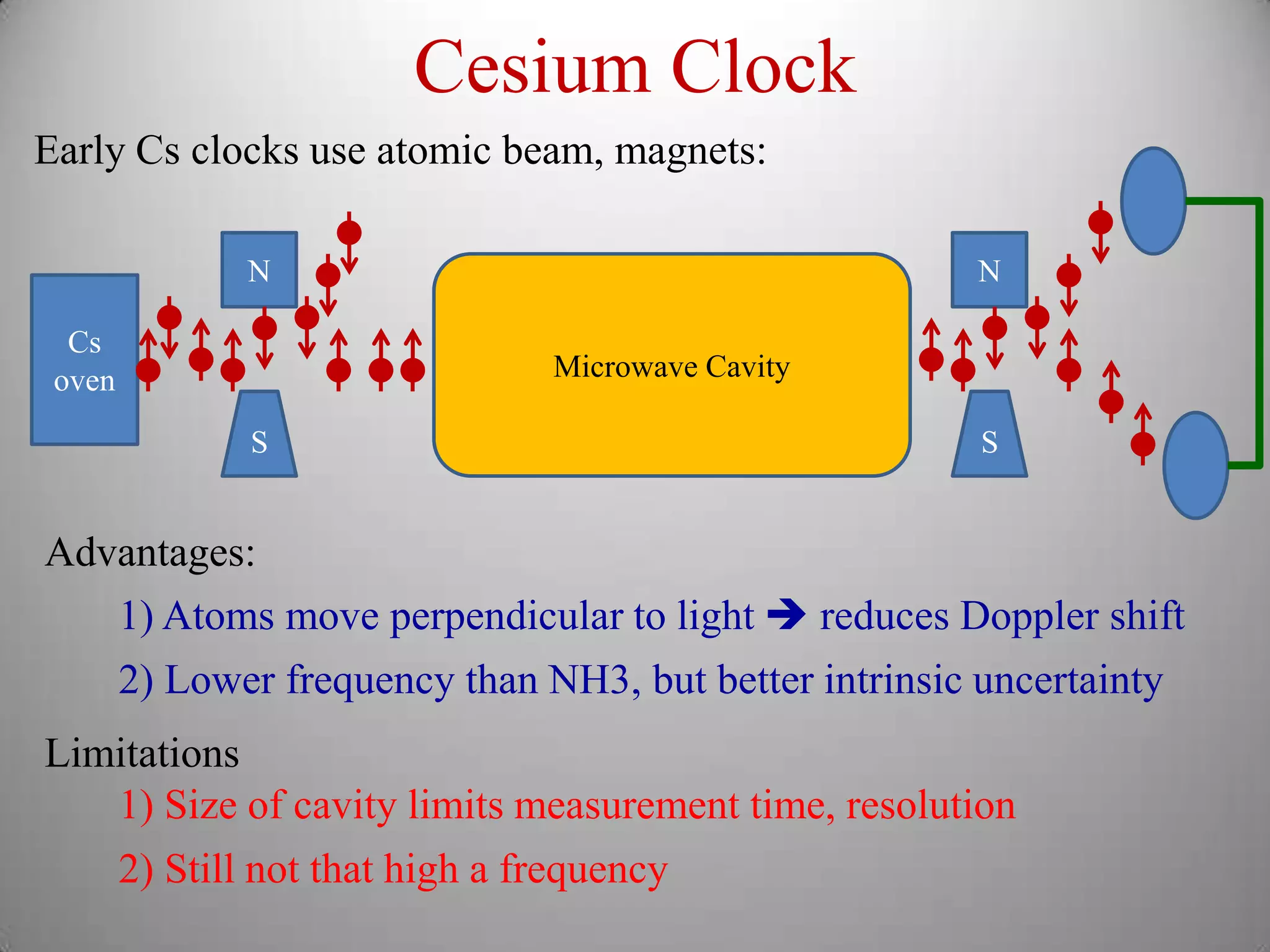
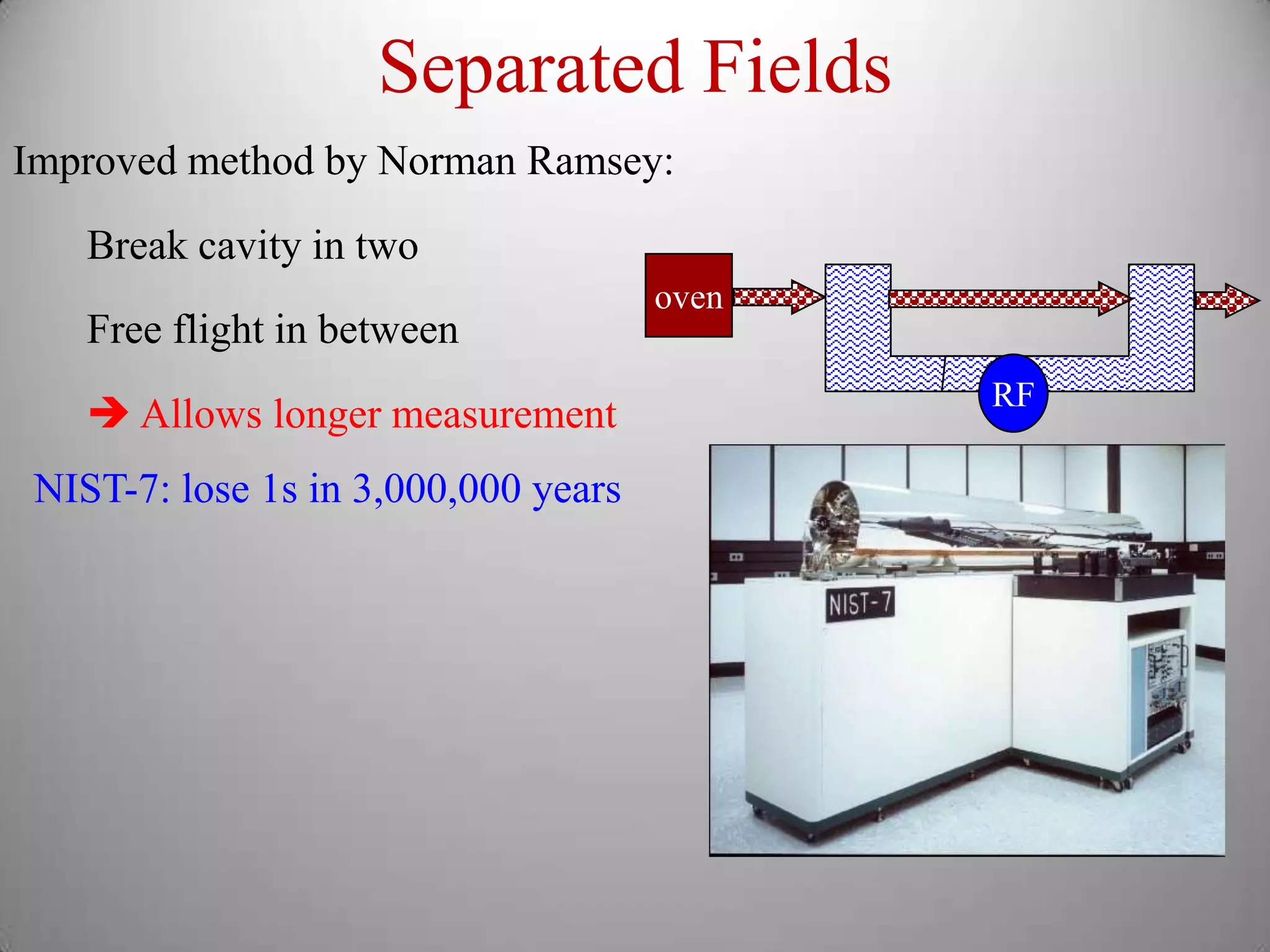
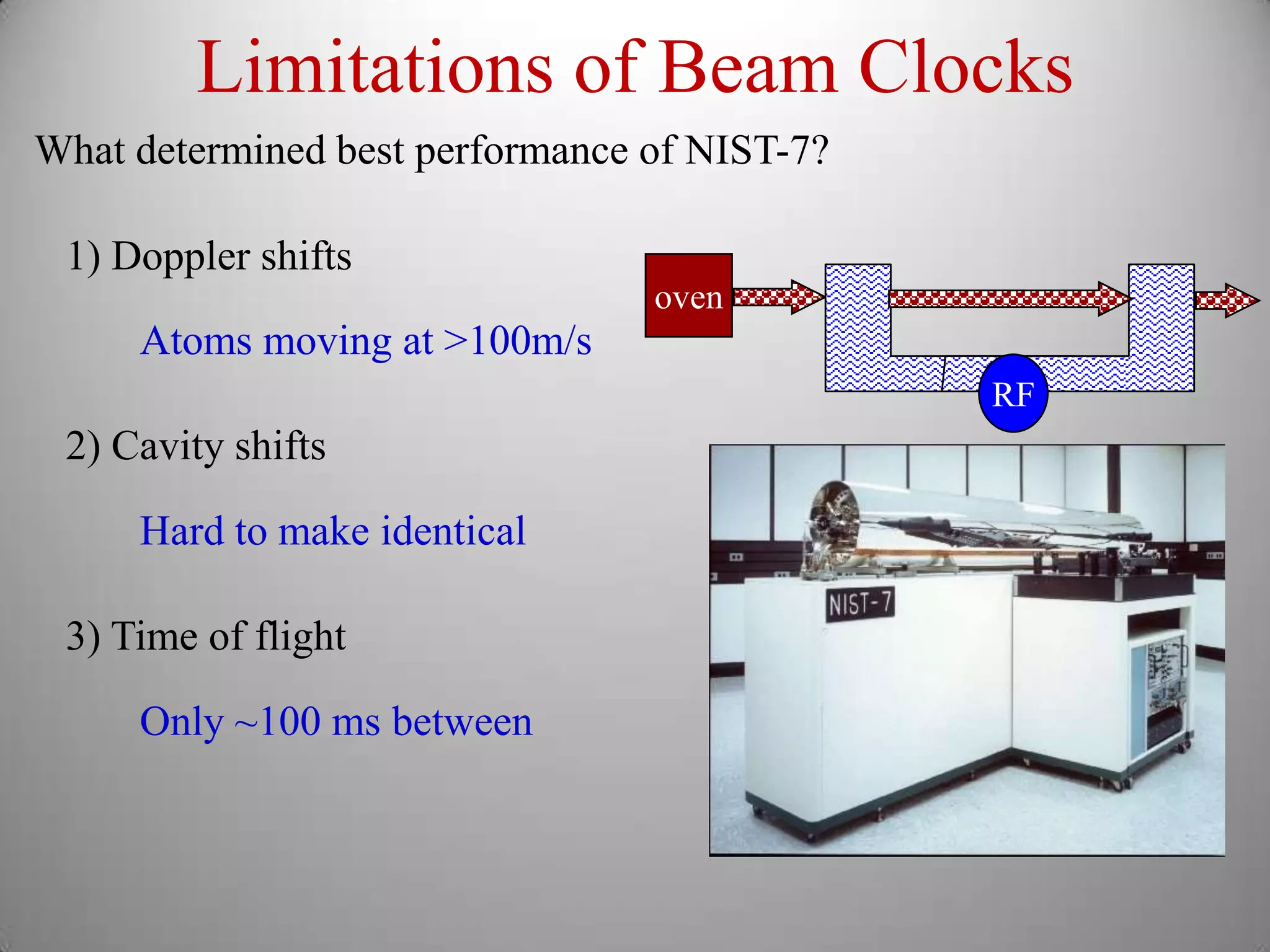
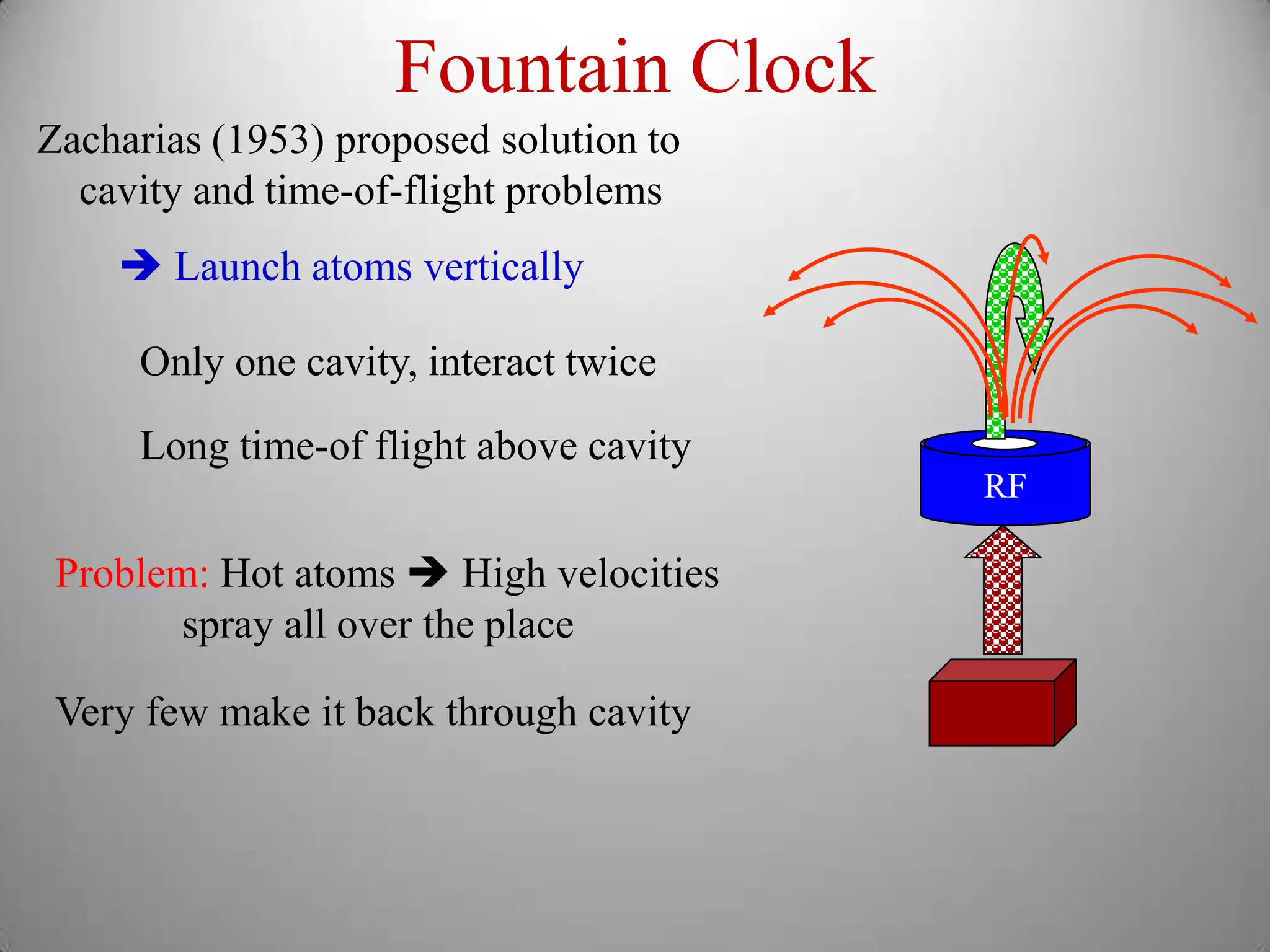
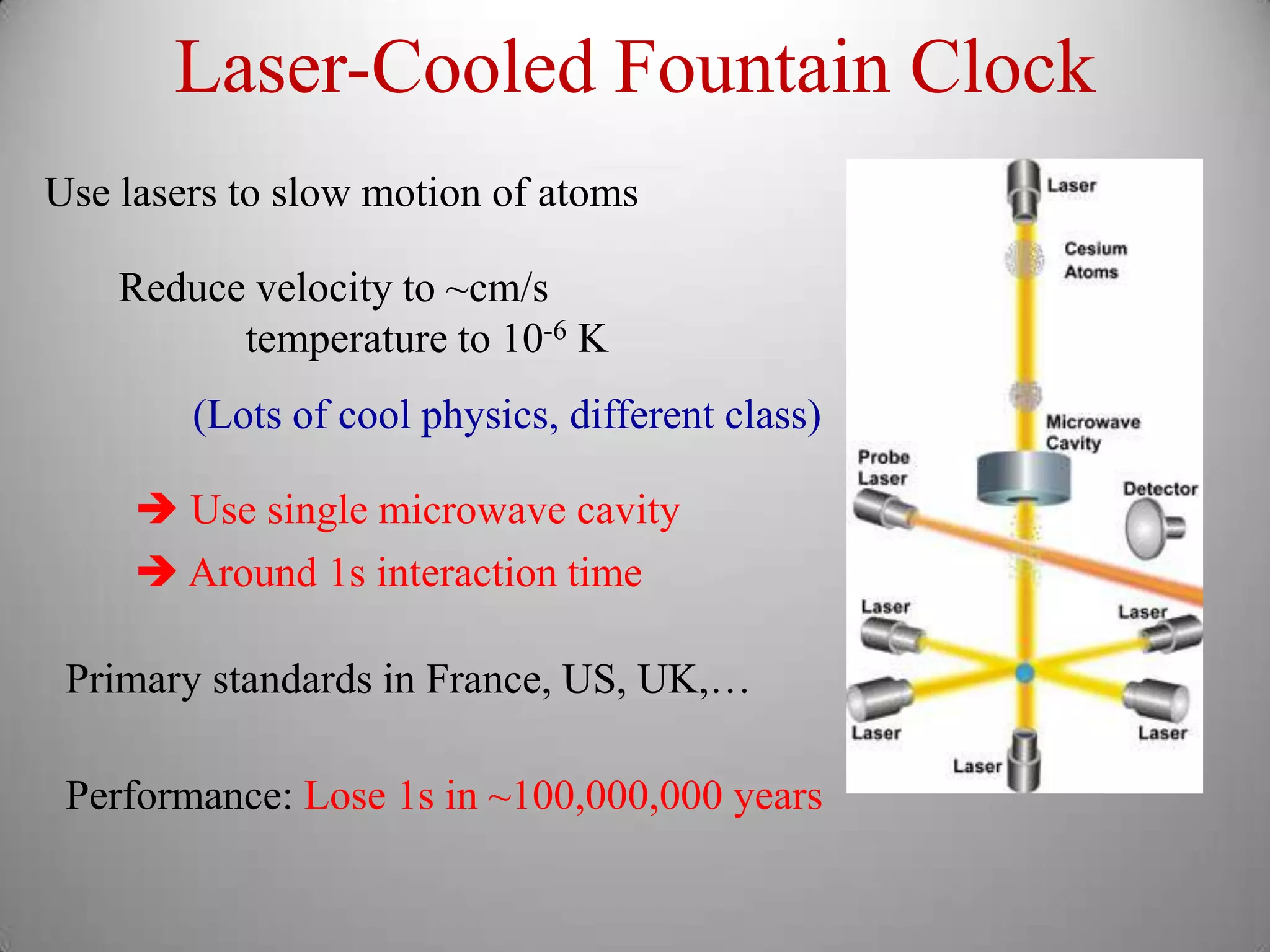
The document discusses key concepts in quantum mechanics, including Planck's hypothesis and the photoelectric effect, which led to significant advancements in understanding atomic and subatomic particles. It details the evolution of time measurement standards, including the introduction of ammonia and cesium atomic clocks, and the quest for higher precision in timekeeping. The text also describes technological improvements like laser-cooled fountain clocks that enhance measurement accuracy, achieving an astonishing loss of just 1 second in 100 million years.