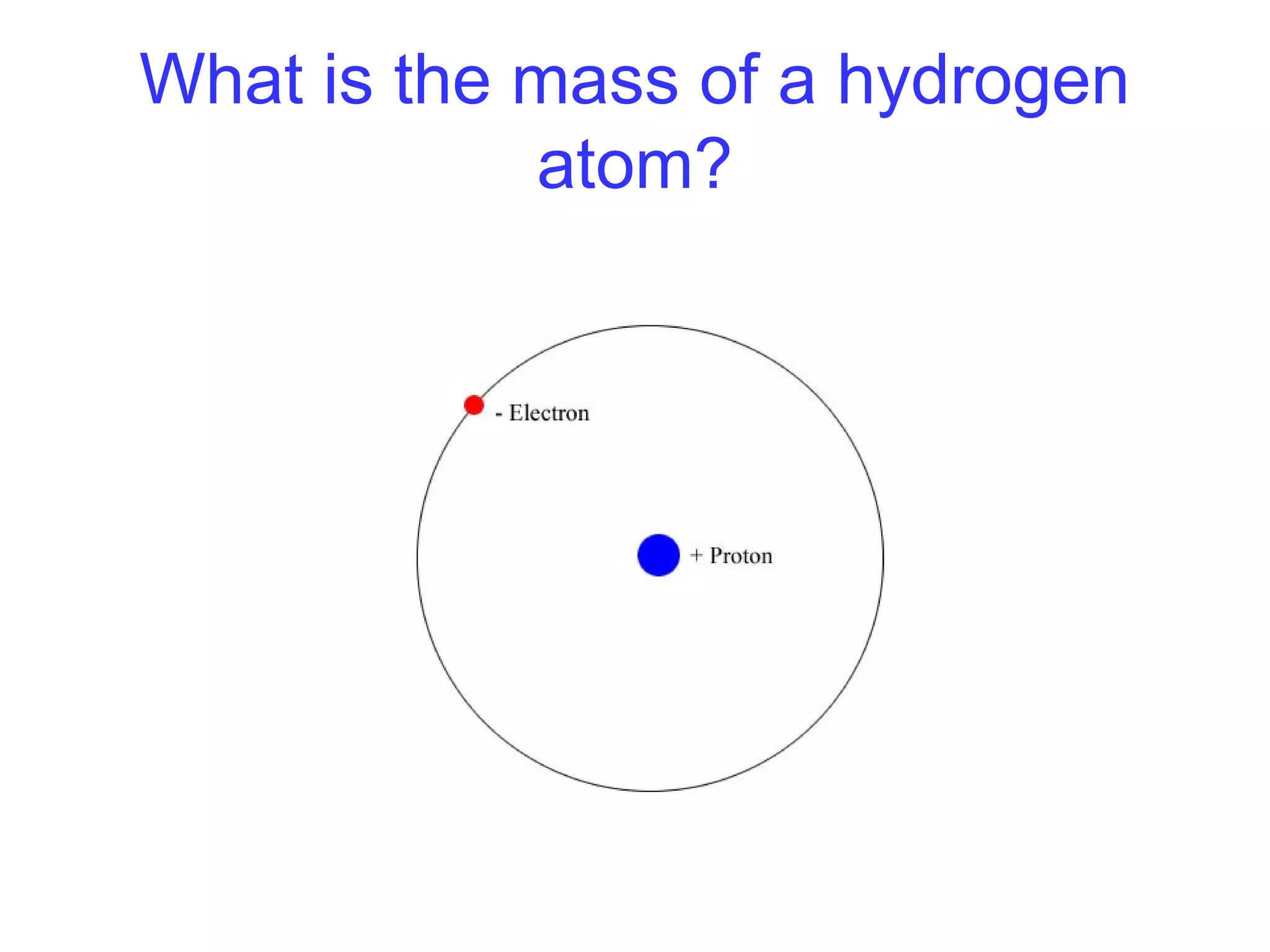
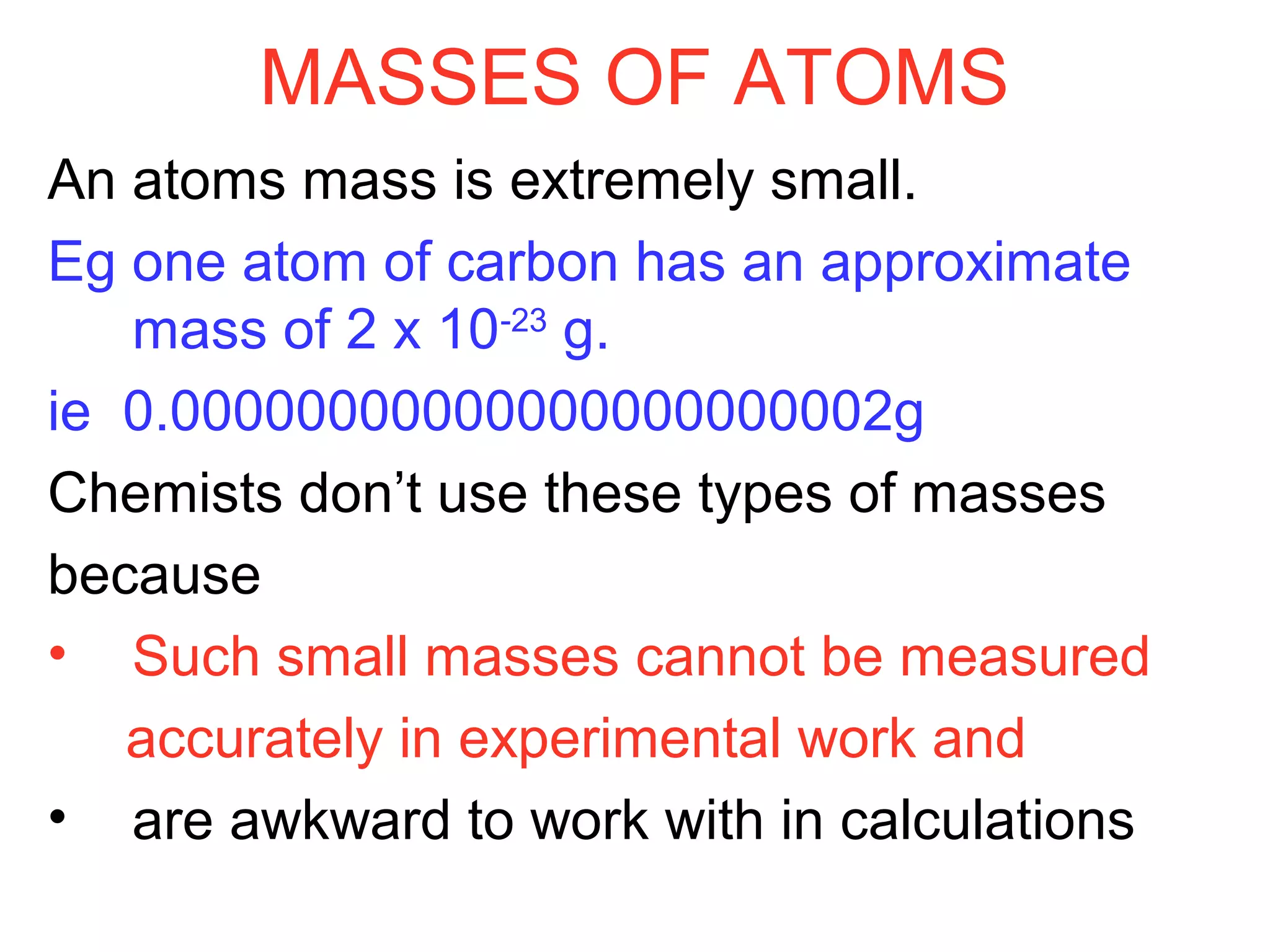
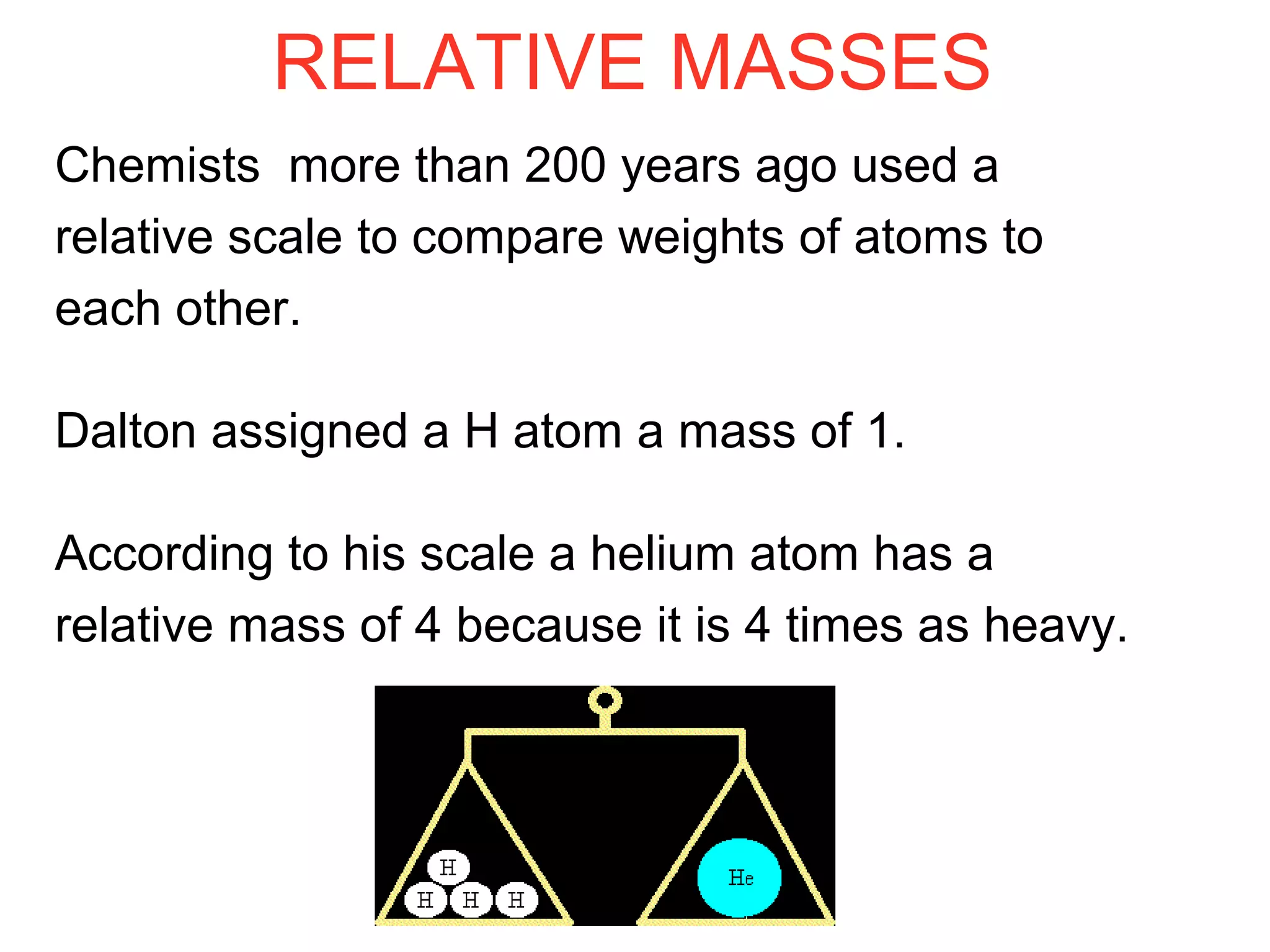
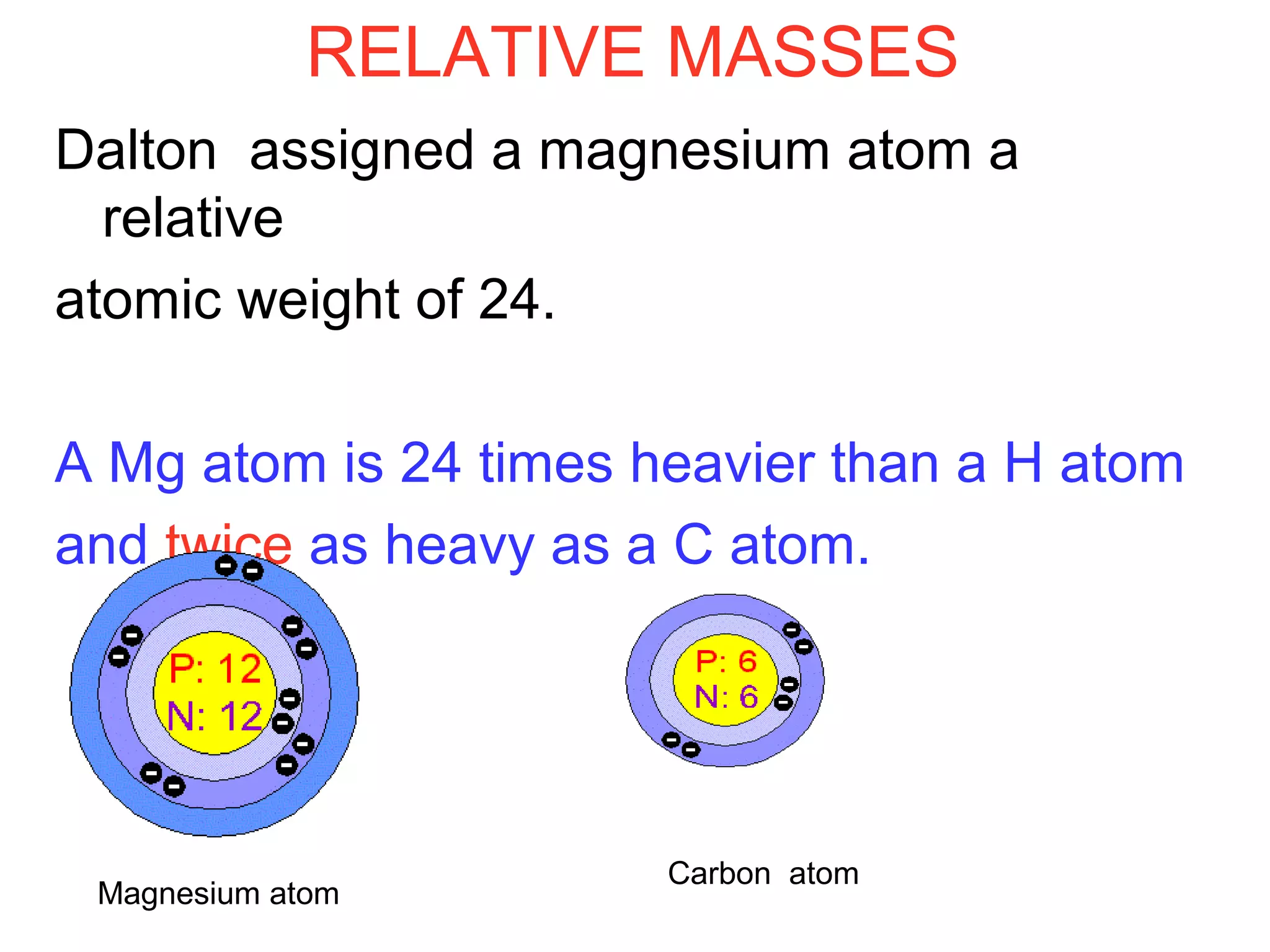
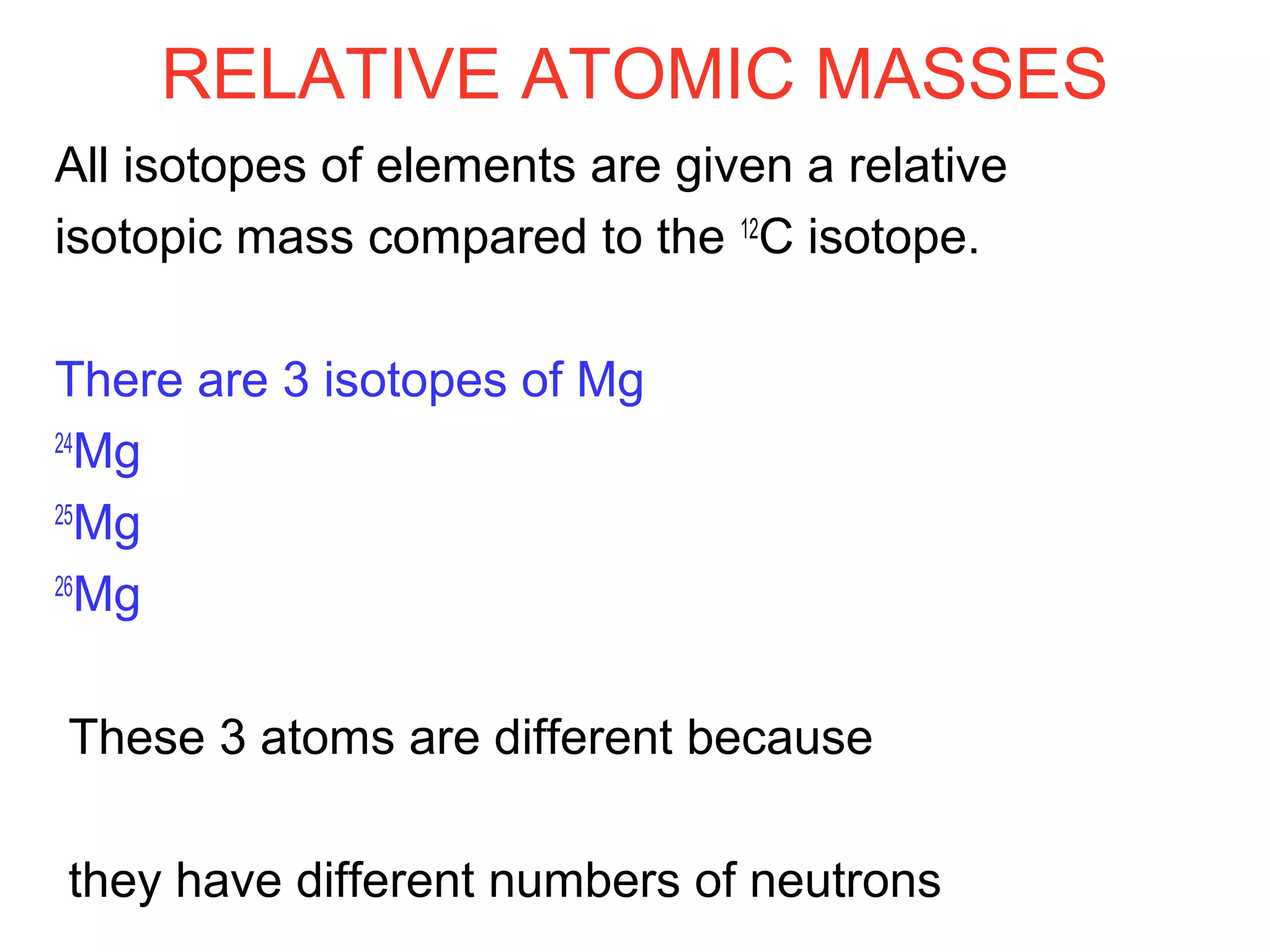
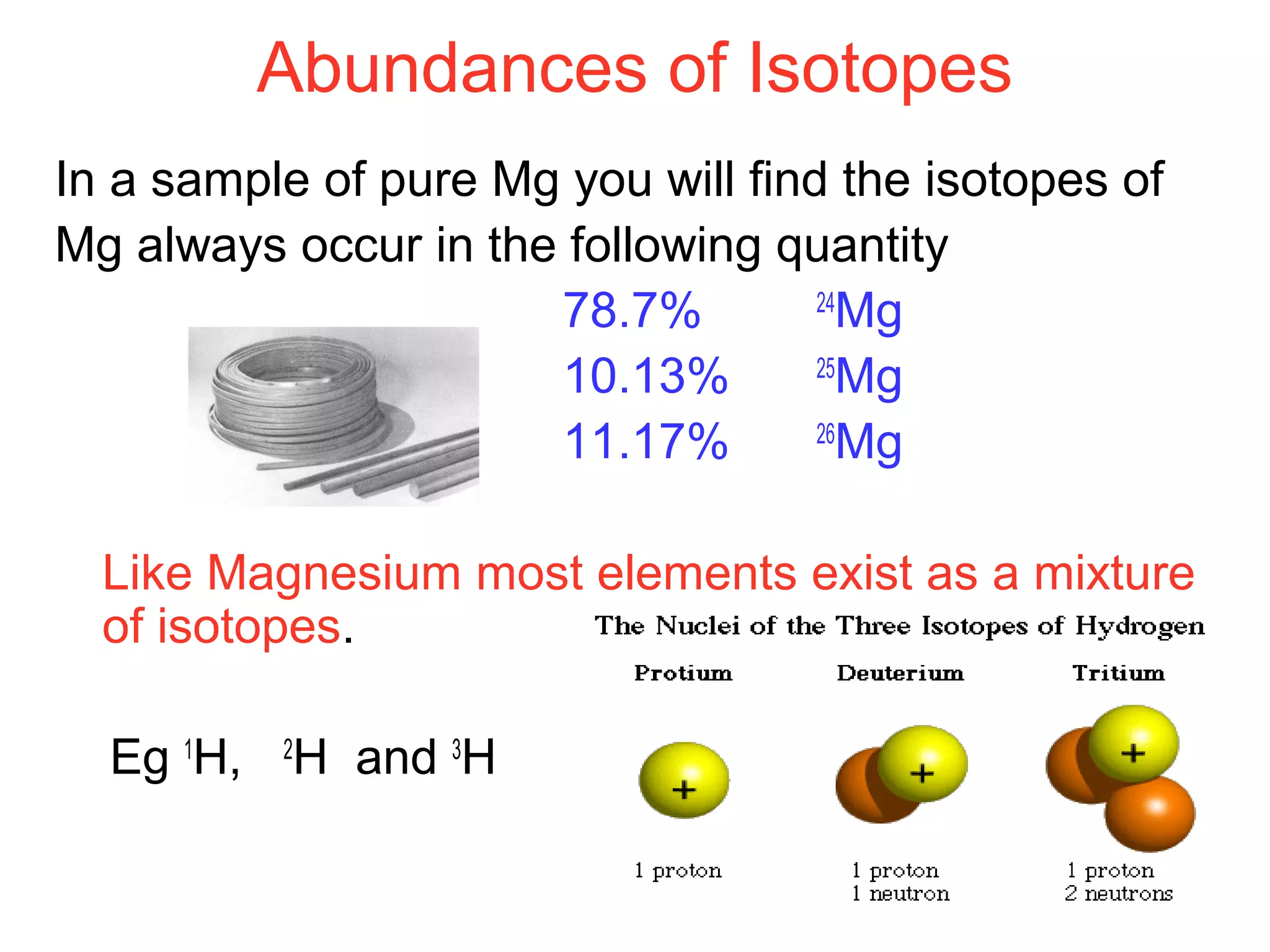
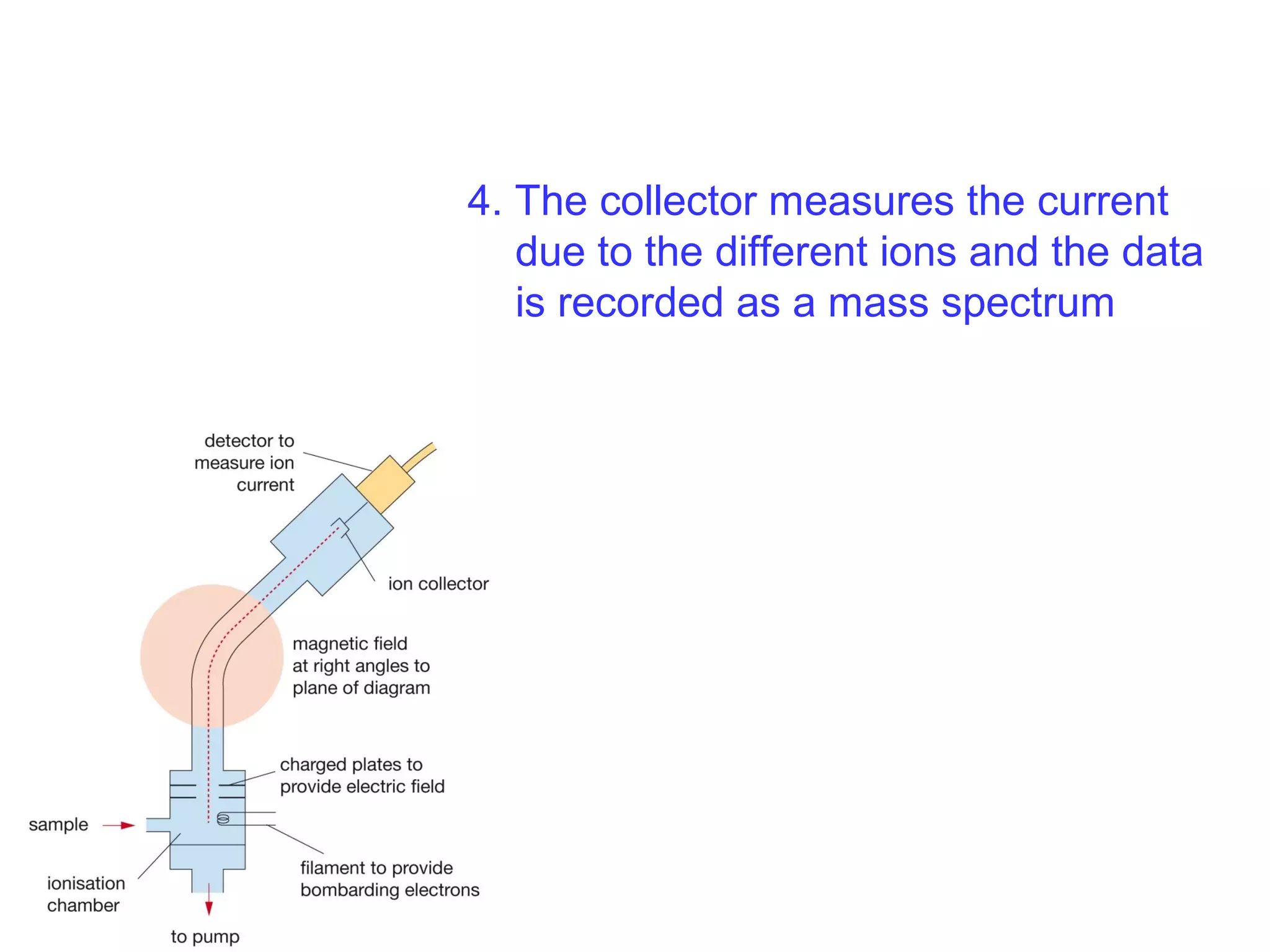
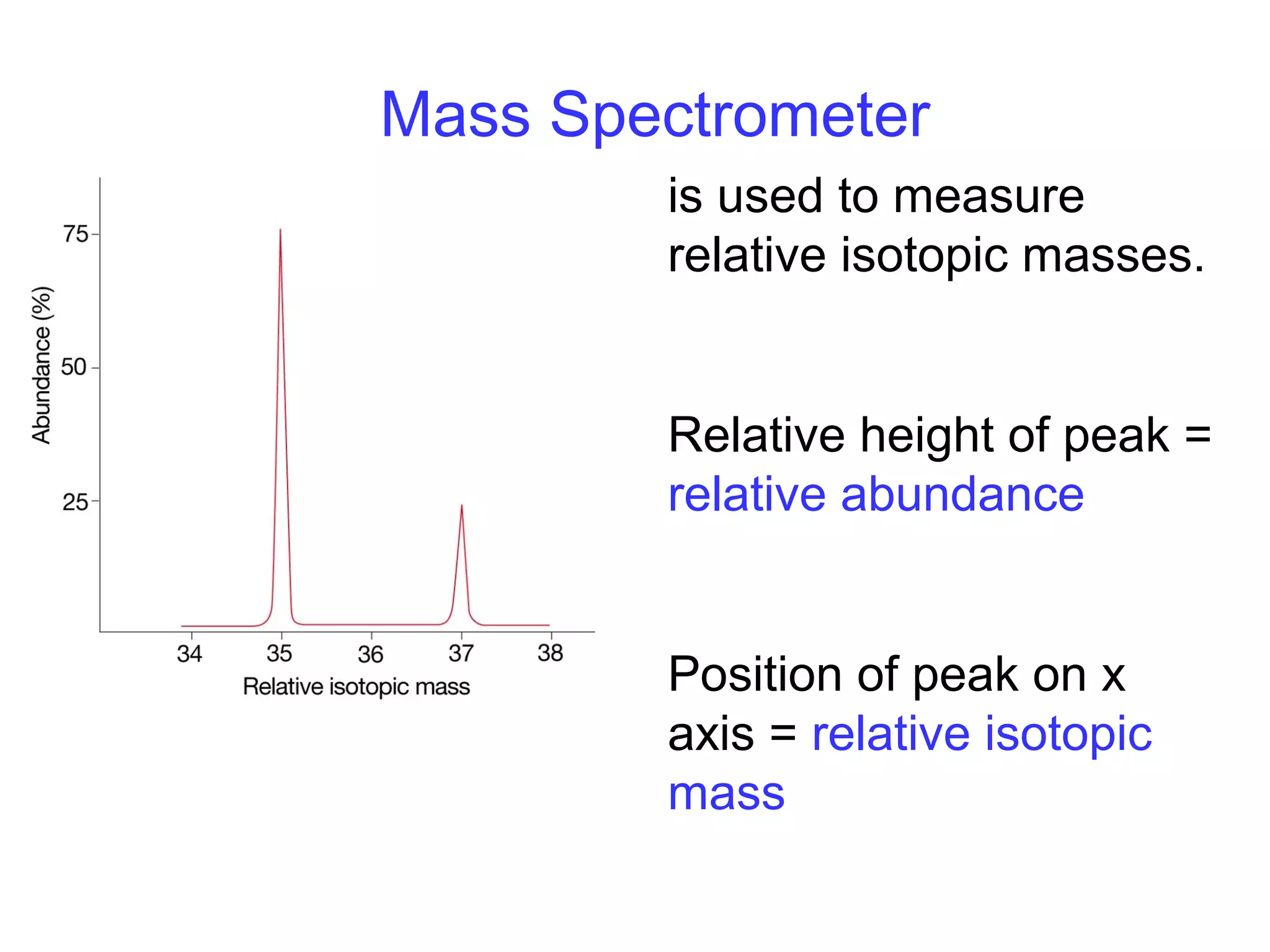
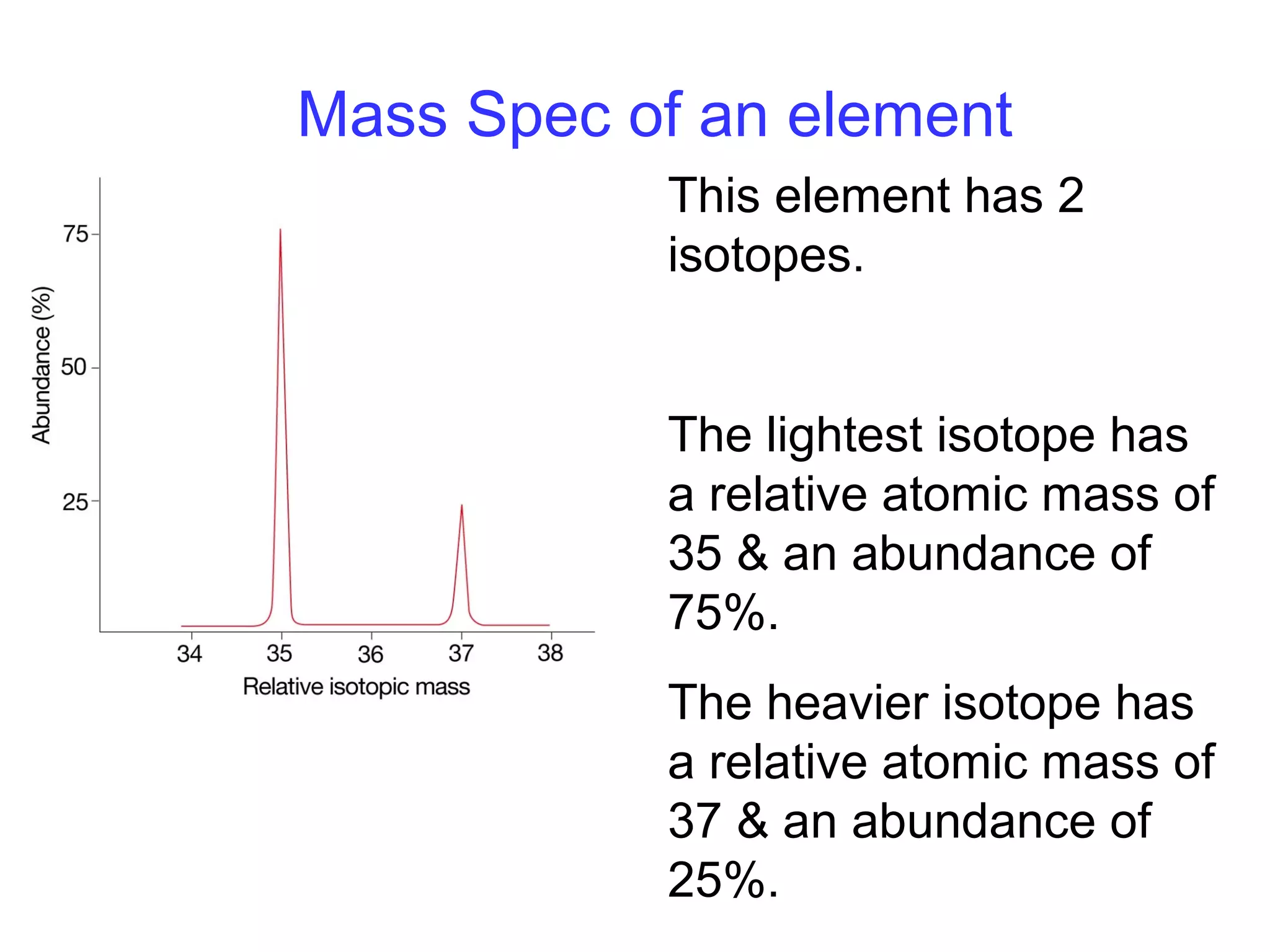
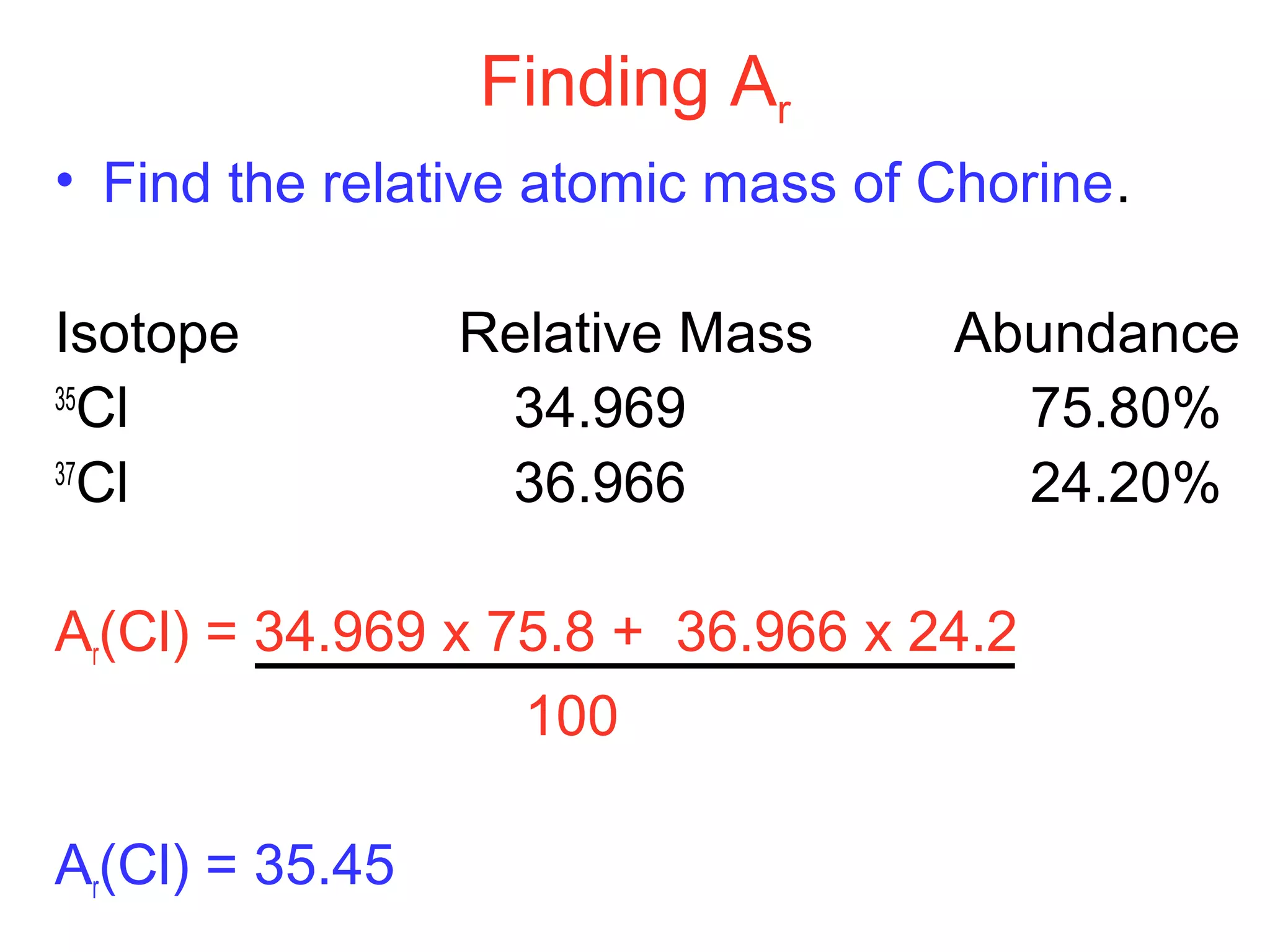
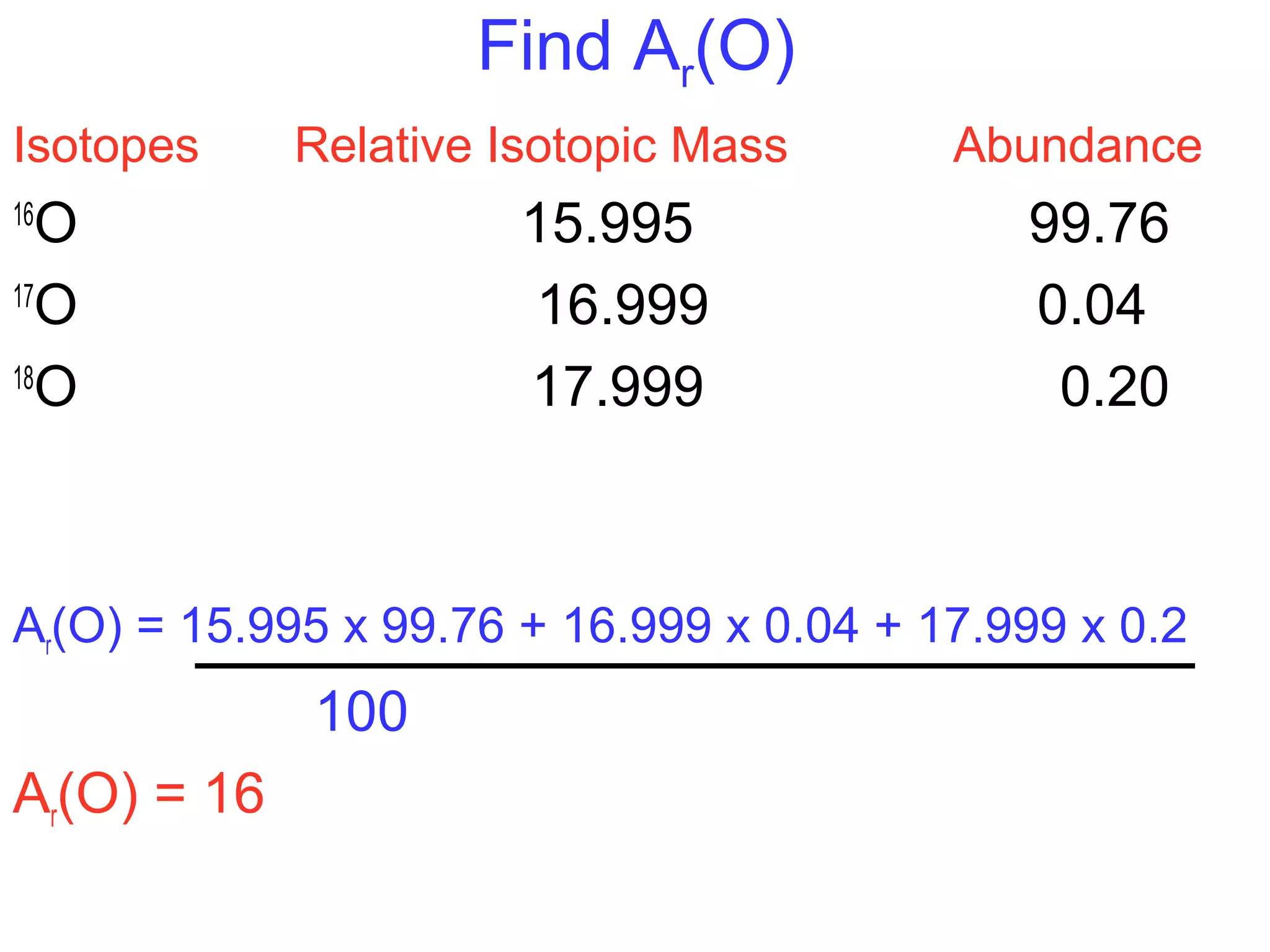
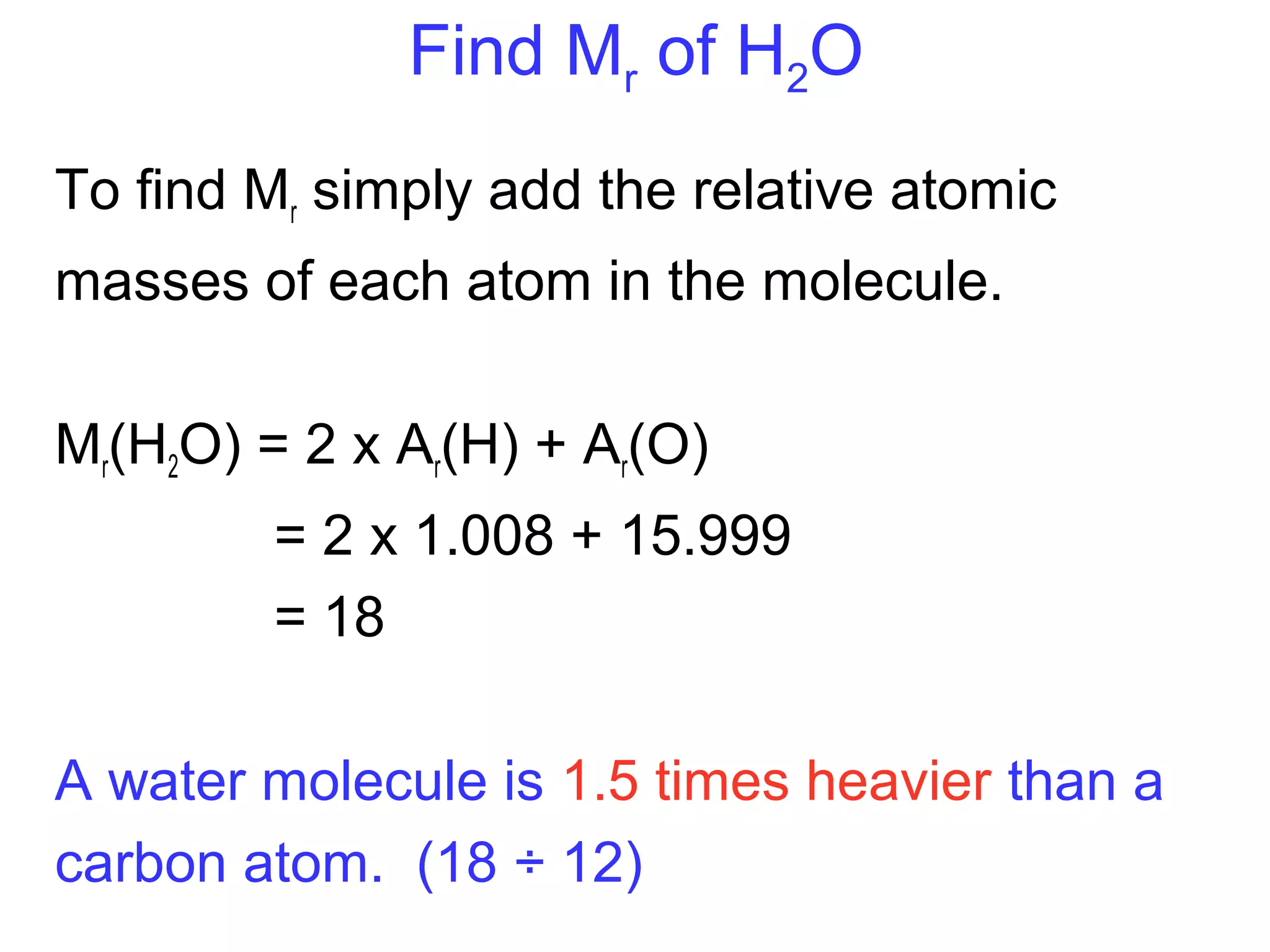
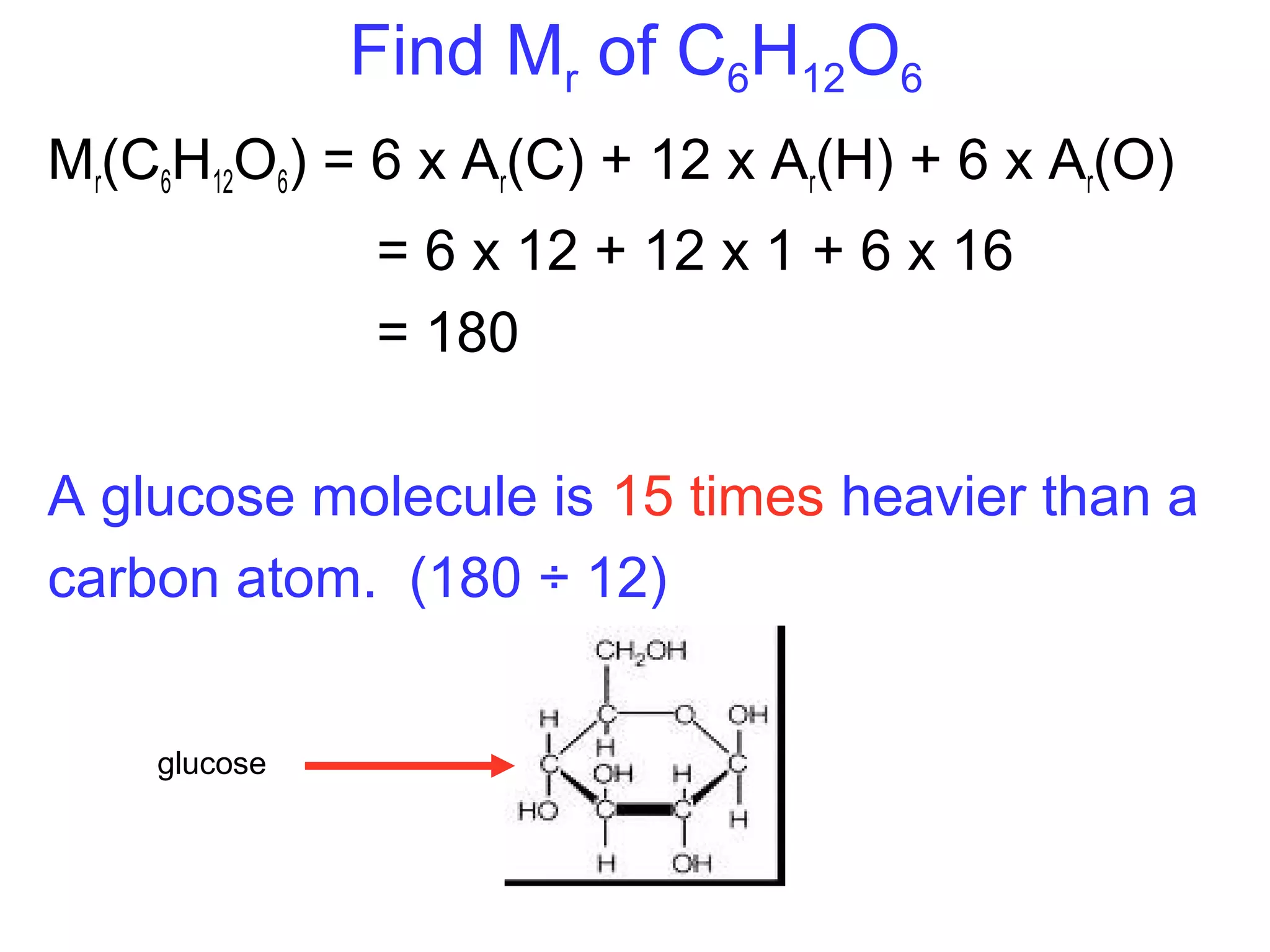
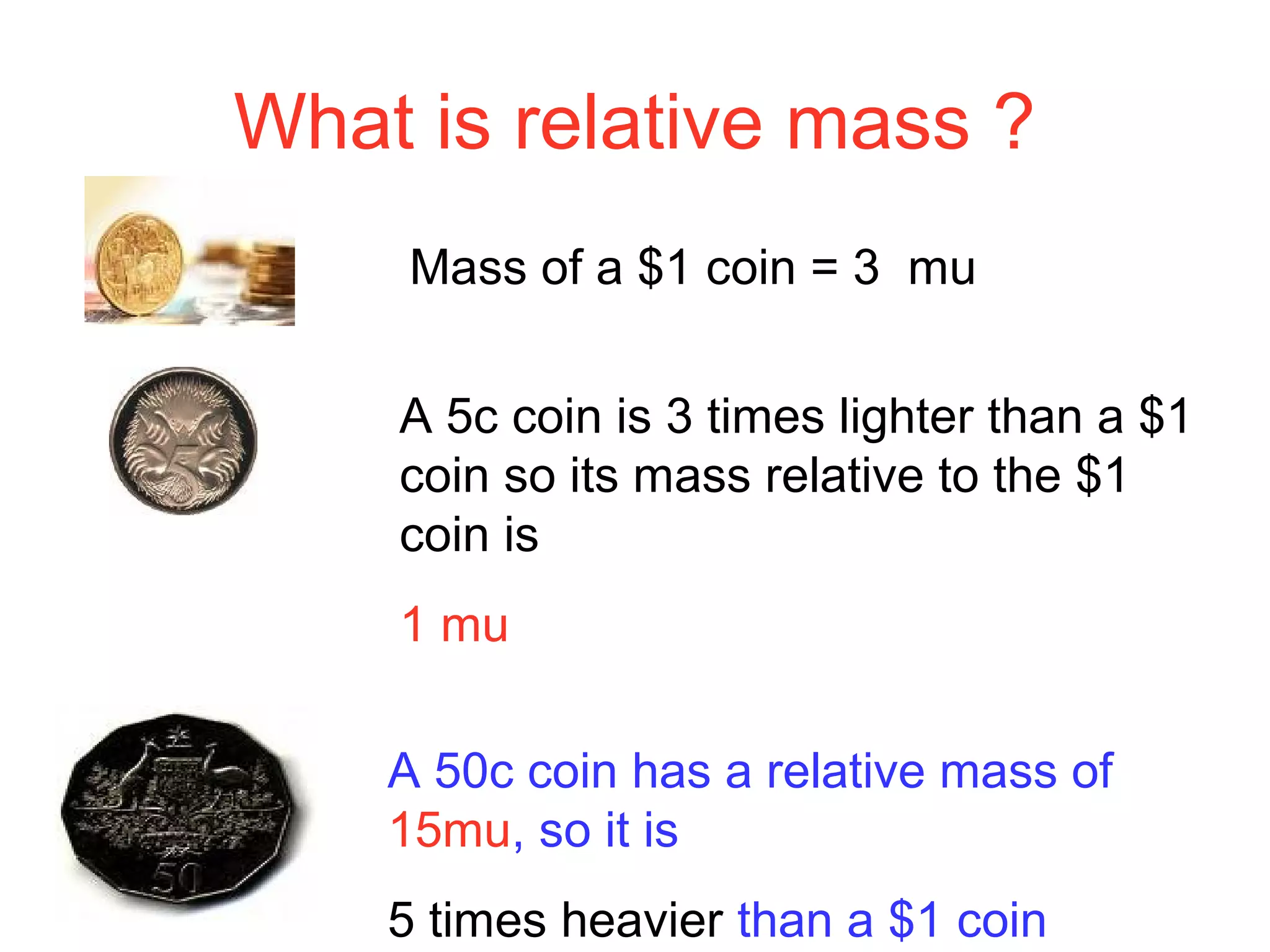This document provides information about relative atomic masses and how they are determined. It discusses how chemists use a relative scale rather than actual atomic masses, which are too small to measure. On this scale, carbon-12 is assigned a mass of 12. Isotopes of each element have different relative isotopic masses. A mass spectrometer is used to separate isotopes and determine their relative abundances. The average relative atomic mass (Ar) of an element is calculated based on the relative isotopic masses and abundances of its isotopes. Molecular and formula masses can also be determined by adding the relative atomic masses of each atom in a molecule or compound unit.