Lower Six Chemistry.pptx
•Download as PPTX, PDF•
0 likes•3 views
Atoms combine to form molecules, and molecules combine to form compounds. Mass spectrometry can determine the relative masses of atoms and molecules. It works by converting samples into ion beams, which are then deflected by electric and magnetic fields based on their mass-to-charge ratio. This allows scientists to determine molecular formulas by identifying molecular fragments and isotope abundances. Stoichiometry uses this information to calculate quantities of materials involved in chemical reactions.
Report
Share
Report
Share
Recommended
atoms and molecules

This document is a chemistry student's report on atoms and molecules. It begins with an introduction discussing the molecular structure hypothesis and how it relates to quantum mechanics. It then covers elemental symbols, compound formulas, the structure of atoms including protons, neutrons, electrons and isotopes. Finally, it discusses atomic mass units, atomic weights, molecular weights, and how the mole concept applies to elements and compounds for chemical calculations. The key topics are represented in less than 3 sentences.
Basic concepts in chemistry

John Dalton proposed the first scientific atomic theory, which stated that each chemical element is composed of atoms of a single, unique type that can combine to form chemical compounds. An atom is the fundamental unit of matter and consists of a nucleus containing protons and neutrons surrounded by electrons. Atoms of the same element contain the same number of protons but can vary in the number of neutrons, resulting in different isotopes of that element. Molecules are the smallest fundamental units of compounds made of two or more atoms bonded together.
Class 9 atom and molecules

class 9 chemistry atom and molecules are explained briefly in a simple language.
Mole concept molar mas etc are described.
Relative atomic mass_&_mass_spectrometry[1][1]![Relative atomic mass_&_mass_spectrometry[1][1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Relative atomic mass_&_mass_spectrometry[1][1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
This document provides information about relative atomic masses and how they are determined. It discusses how chemists use a relative scale rather than actual atomic masses, which are too small to measure. On this scale, carbon-12 is assigned a mass of 12. Isotopes of each element have different relative isotopic masses. A mass spectrometer is used to separate isotopes and determine their relative abundances. The average relative atomic mass (Ar) of an element is calculated based on the relative isotopic masses and abundances of its isotopes. Molecular and formula masses can also be determined by adding the relative atomic masses of each atom in a molecule or compound unit.
Chemistry Lecture Slide Week 1

This lecture covers the basics of matter including the three phases of matter, classification of matter as pure substances or mixtures, and the key differences between elements, compounds, and mixtures. It also discusses atomic structure including atoms, ions, isotopes, and the atomic mass scale. The lecture defines important terms like atomic number, mass number, relative atomic mass. It describes how to name ionic and molecular compounds using common nomenclature rules. Key topics covered include Dalton's atomic theory, the mass spectrometer, and different families of ions like oxoanions.
Chem 111 Chapter 2: Ions atoms and molecules 

General chemistry I chapter 2: Learn about Ions, Atoms, and Molecules. The building blocks of matter. Pace yourself and stay ahead of the game!
Mass spectrometry

Mass spectrometry is a technique that measures the mass-to-charge ratio of ions. It works by ionizing analyte molecules, then separating the ions based on their m/z ratios using electric and magnetic fields. The data produced are mass spectra, which provide information about molecular structure. Key developments included techniques like electron ionization, electrospray ionization, and matrix-assisted laser desorption/ionization, which allowed analysis of nonvolatile and thermally labile compounds. Mass spectrometry determines isotopic composition and is used to study elemental, molecular, and isotopic properties of substances.
Some basic concept of chemistry 2017

Chemistry is the study of matter and its interactions. The basic units that make up all matter are atoms, which combine to form molecules. Atoms are made up of protons, neutrons, and electrons. The number of protons in an atom determines its atomic number, while the total mass of an atom is determined by its atomic mass number. Molecules are combinations of atoms that are bonded together chemically. The mole is a unit used to measure amounts of substances, equal to 6.022x1023 particles. Stoichiometry uses mole ratios to calculate amounts of reactants and products in chemical reactions.
Recommended
atoms and molecules

This document is a chemistry student's report on atoms and molecules. It begins with an introduction discussing the molecular structure hypothesis and how it relates to quantum mechanics. It then covers elemental symbols, compound formulas, the structure of atoms including protons, neutrons, electrons and isotopes. Finally, it discusses atomic mass units, atomic weights, molecular weights, and how the mole concept applies to elements and compounds for chemical calculations. The key topics are represented in less than 3 sentences.
Basic concepts in chemistry

John Dalton proposed the first scientific atomic theory, which stated that each chemical element is composed of atoms of a single, unique type that can combine to form chemical compounds. An atom is the fundamental unit of matter and consists of a nucleus containing protons and neutrons surrounded by electrons. Atoms of the same element contain the same number of protons but can vary in the number of neutrons, resulting in different isotopes of that element. Molecules are the smallest fundamental units of compounds made of two or more atoms bonded together.
Class 9 atom and molecules

class 9 chemistry atom and molecules are explained briefly in a simple language.
Mole concept molar mas etc are described.
Relative atomic mass_&_mass_spectrometry[1][1]![Relative atomic mass_&_mass_spectrometry[1][1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Relative atomic mass_&_mass_spectrometry[1][1]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
This document provides information about relative atomic masses and how they are determined. It discusses how chemists use a relative scale rather than actual atomic masses, which are too small to measure. On this scale, carbon-12 is assigned a mass of 12. Isotopes of each element have different relative isotopic masses. A mass spectrometer is used to separate isotopes and determine their relative abundances. The average relative atomic mass (Ar) of an element is calculated based on the relative isotopic masses and abundances of its isotopes. Molecular and formula masses can also be determined by adding the relative atomic masses of each atom in a molecule or compound unit.
Chemistry Lecture Slide Week 1

This lecture covers the basics of matter including the three phases of matter, classification of matter as pure substances or mixtures, and the key differences between elements, compounds, and mixtures. It also discusses atomic structure including atoms, ions, isotopes, and the atomic mass scale. The lecture defines important terms like atomic number, mass number, relative atomic mass. It describes how to name ionic and molecular compounds using common nomenclature rules. Key topics covered include Dalton's atomic theory, the mass spectrometer, and different families of ions like oxoanions.
Chem 111 Chapter 2: Ions atoms and molecules 

General chemistry I chapter 2: Learn about Ions, Atoms, and Molecules. The building blocks of matter. Pace yourself and stay ahead of the game!
Mass spectrometry

Mass spectrometry is a technique that measures the mass-to-charge ratio of ions. It works by ionizing analyte molecules, then separating the ions based on their m/z ratios using electric and magnetic fields. The data produced are mass spectra, which provide information about molecular structure. Key developments included techniques like electron ionization, electrospray ionization, and matrix-assisted laser desorption/ionization, which allowed analysis of nonvolatile and thermally labile compounds. Mass spectrometry determines isotopic composition and is used to study elemental, molecular, and isotopic properties of substances.
Some basic concept of chemistry 2017

Chemistry is the study of matter and its interactions. The basic units that make up all matter are atoms, which combine to form molecules. Atoms are made up of protons, neutrons, and electrons. The number of protons in an atom determines its atomic number, while the total mass of an atom is determined by its atomic mass number. Molecules are combinations of atoms that are bonded together chemically. The mole is a unit used to measure amounts of substances, equal to 6.022x1023 particles. Stoichiometry uses mole ratios to calculate amounts of reactants and products in chemical reactions.
PPT ATOMS AND MOLECULE 

This document provides an overview of atoms and molecules. It defines key terms like atom, molecule, ion, and discusses Dalton's atomic theory and its postulates. The document explains that atoms are the smallest particles that make up matter and combine to form molecules or ions. It discusses how elements are represented by symbols and how atomic mass is measured in atomic mass units relative to carbon-12. The document also summarizes laws of chemical combination and provides examples of writing chemical formulas based on valencies of elements.
_kalvi_11t.pptx

This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types, while compounds contain two or more different atom types bonded together. The mole concept allows chemists to quantify amounts of substances down to the molecular level. Chemical calculations can be performed based on molar mass, stoichiometry from balanced equations, and other relationships between amounts of reactants and products.
Chapter 3

First Year in Dentistry First Semester
Al-Azhar University - Gaza
Lama El Banna
general chemistry
Laws of Chemical Combination + Stoichiometry

The document discusses several important laws and concepts in chemistry:
1) The Law of Constant Composition states that a chemical compound always contains the same elements in the same proportions by mass.
2) The Law of Conservation of Mass says that mass is neither created nor destroyed in chemical reactions.
3) The Law of Multiple Proportions states that when two elements form more than one compound, the ratios of the masses of one element that combine with a fixed mass of the other element are ratios of small whole numbers.
4) Concepts like molar mass, the mole, and stoichiometry provide quantitative relationships between substances in chemical equations and reactions.
11th_chemistry_unit_1_ppt_em_218218.pptx

This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types that can be monatomic or polyatomic. Compounds contain two or more elements. The mole concept is introduced to quantify atoms and molecules using Avogadro's number. Empirical and molecular formulas are distinguished. Methods for determining formulas from elemental analysis or molar mass are presented. Equivalent mass and stoichiometric calculations are also covered.
namma_kalvi_11th_chemistry_unit_1_ppt_em_218218.pptx

This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types that can be monatomic or polyatomic. Compounds contain two or more elements. The mole concept is introduced to quantify atoms and molecules using Avogadro's number of 6.022x1023 particles. Empirical and molecular formulas are distinguished. Methods are described for determining empirical formulas from elemental analysis data and calculating molecular formulas. Stoichiometry allows determining quantitative relationships between reactants and products in chemical equations
some basic concepts of chem notes .pdf

1. The document provides an overview of basic concepts in chemistry covered in an 11th grade chemistry course, including the divisions of chemistry, laws of chemical combination, Dalton's atomic theory, significant figures, stoichiometric coefficients, the mole, Avogadro's number, and the limiting reagent.
2. Key concepts are explained, such as how the limiting reagent determines how much of a product can be formed in a chemical reaction.
3. Example problems are provided to illustrate calculating molarity, empirical and molecular formulas, and amount of products formed using stoichiometric calculations and identifying the limiting reagent.
Introduction To Chemistry Power Point

This document provides an introduction to basic chemistry concepts including the structure of atoms, atomic mass, and quantities. Atoms consist of a dense positively charged nucleus made up of protons and neutrons surrounded by an electron cloud of negatively charged electrons. The number of protons determines the element. Atomic mass is measured in atomic mass units and is based on the number of protons and neutrons. Elements can have different isotopes that have the same number of protons but different numbers of neutrons, resulting in different atomic masses. Quantities in chemistry such as moles and molarity are also introduced.
ATOMS, MOLECULES AND IONS

The document discusses the atomic theory of matter and the development of atomic structure models. It describes John Dalton's atomic theory which stated that elements are composed of atoms that are unique and atoms are neither created nor destroyed in chemical reactions. The discovery of the electron by J.J. Thompson and experiments by Robert Millikan and Ernest Rutherford helped develop the modern atomic structure model of a small, dense nucleus surrounded by electrons. The document also discusses isotopes, atomic numbers, mass numbers, and how the periodic table is arranged based on atomic structure.
CH2 (1).pdf

This document provides an overview of atomic structure and bonding. It begins by reviewing John Dalton's atomic theory from 1808. It then discusses experiments that revealed subatomic particles like electrons, protons, and neutrons, leading to Rutherford's nuclear model of the atom. The document explains atomic number, mass number, and isotopes. It also introduces the periodic table and how it is arranged based on atomic structure. Finally, it distinguishes between molecular compounds formed by covalent bonds and ionic compounds formed by ionic bonds.
CHEM1 NOTES, B.pptx

This document provides information about chemistry concepts including the atomic theory, structure of the atom, atomic mass units, atomic weights, elements, compounds, and pure substances vs mixtures. Key points include:
- John Dalton formulated the atomic theory including that atoms are indivisible and differ between elements.
- Rutherford discovered the nuclear model of the atom with a small, dense nucleus and electrons in empty space around it.
- Atoms contain protons, neutrons, and electrons. Electrons are arranged in shells around the nucleus.
- Atomic mass units are used to express atomic masses rather than grams due to atoms' small size. Atomic weights are relative masses of atoms compared to carbon-12.
Atomic Theory.pptx

Rutherford's gold foil experiment involved firing alpha particles at a thin sheet of gold foil and observing their scattering. The key findings were:
- The nucleus is small and dense
- It is positively charged
- Most alpha particles passed through the foil with little deflection, but a few were greatly deflected, surprising Rutherford.
This led to the conclusion that atoms have a small, dense, positively charged nucleus surrounded by orbiting electrons. The modern atomic model consists of protons and neutrons in the nucleus surrounded by electrons in an electron cloud.
Amount of substance

This document discusses the mole concept and stoichiometry calculations. It defines key terms like the mole, molar mass, empirical and molecular formulas, and limiting reagents. It also describes how to use balanced chemical equations and molar relationships to calculate amounts of reactants and products involved in chemical reactions. Experimental procedures are provided for determining relative molecular masses and identifying unknown metals based on stoichiometric calculations.
Ch02 lecture

Here are the steps to complete the table:
1. Atomic number = number of protons
2. For neutral atoms, number of electrons = number of protons
3. Mass number = number of protons + number of neutrons
4. Atomic symbol is the element symbol
So the completed table would be:
Protons Neutrons Electrons Atomic Number Mass Number Atomic Symb
8 8 8 8 16 O
13 13 13 13 26 Fe
79 79 X 79 79 158 Au
The only unknown is the number of neutrons, which is calculated by taking the mass number and subtracting the number of protons.
Grade 9, U1-L9-Atomic structure

This document discusses the structure of atoms and their subatomic particles. It explains that atoms are made up of protons, neutrons, and electrons. Protons and neutrons are located in the nucleus at the center of the atom, while electrons surround the nucleus in energy levels. The number of protons determines the element and is known as the atomic number. The total number of protons and neutrons is the mass number. Standard atomic notation uses the mass number as a superscript on the left of the element symbol and the atomic number as a subscript.
Chapter 4 pp (1)

This document summarizes key concepts about atomic structure:
1) It describes early atomic models including Democritus' idea of indivisible atoms and Dalton's atomic theory.
2) It explains the discovery of subatomic particles (electrons, protons, neutrons) and the nuclear model of the atom with electrons orbiting a nucleus.
3) It defines important atomic properties including atomic number, mass number, isotopes, and how to calculate atomic mass.
4) It provides an overview of how the periodic table organizes elements based on these atomic properties.
The cost of acquiring information by natural selection

This is a short talk that I gave at the Banff International Research Station workshop on Modeling and Theory in Population Biology. The idea is to try to understand how the burden of natural selection relates to the amount of information that selection puts into the genome.
It's based on the first part of this research paper:
The cost of information acquisition by natural selection
Ryan Seamus McGee, Olivia Kosterlitz, Artem Kaznatcheev, Benjamin Kerr, Carl T. Bergstrom
bioRxiv 2022.07.02.498577; doi: https://doi.org/10.1101/2022.07.02.498577
More Related Content
Similar to Lower Six Chemistry.pptx
PPT ATOMS AND MOLECULE 

This document provides an overview of atoms and molecules. It defines key terms like atom, molecule, ion, and discusses Dalton's atomic theory and its postulates. The document explains that atoms are the smallest particles that make up matter and combine to form molecules or ions. It discusses how elements are represented by symbols and how atomic mass is measured in atomic mass units relative to carbon-12. The document also summarizes laws of chemical combination and provides examples of writing chemical formulas based on valencies of elements.
_kalvi_11t.pptx

This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types, while compounds contain two or more different atom types bonded together. The mole concept allows chemists to quantify amounts of substances down to the molecular level. Chemical calculations can be performed based on molar mass, stoichiometry from balanced equations, and other relationships between amounts of reactants and products.
Chapter 3

First Year in Dentistry First Semester
Al-Azhar University - Gaza
Lama El Banna
general chemistry
Laws of Chemical Combination + Stoichiometry

The document discusses several important laws and concepts in chemistry:
1) The Law of Constant Composition states that a chemical compound always contains the same elements in the same proportions by mass.
2) The Law of Conservation of Mass says that mass is neither created nor destroyed in chemical reactions.
3) The Law of Multiple Proportions states that when two elements form more than one compound, the ratios of the masses of one element that combine with a fixed mass of the other element are ratios of small whole numbers.
4) Concepts like molar mass, the mole, and stoichiometry provide quantitative relationships between substances in chemical equations and reactions.
11th_chemistry_unit_1_ppt_em_218218.pptx

This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types that can be monatomic or polyatomic. Compounds contain two or more elements. The mole concept is introduced to quantify atoms and molecules using Avogadro's number. Empirical and molecular formulas are distinguished. Methods for determining formulas from elemental analysis or molar mass are presented. Equivalent mass and stoichiometric calculations are also covered.
namma_kalvi_11th_chemistry_unit_1_ppt_em_218218.pptx

This document provides an overview of basic chemistry concepts and calculations. It begins by explaining that chemistry is involved in everyday life through materials like polymers, drugs, and alloys. Matter can be classified based on physical state as solid, liquid, or gas, and chemically as pure substances or mixtures. Elements are composed of single atom types that can be monatomic or polyatomic. Compounds contain two or more elements. The mole concept is introduced to quantify atoms and molecules using Avogadro's number of 6.022x1023 particles. Empirical and molecular formulas are distinguished. Methods are described for determining empirical formulas from elemental analysis data and calculating molecular formulas. Stoichiometry allows determining quantitative relationships between reactants and products in chemical equations
some basic concepts of chem notes .pdf

1. The document provides an overview of basic concepts in chemistry covered in an 11th grade chemistry course, including the divisions of chemistry, laws of chemical combination, Dalton's atomic theory, significant figures, stoichiometric coefficients, the mole, Avogadro's number, and the limiting reagent.
2. Key concepts are explained, such as how the limiting reagent determines how much of a product can be formed in a chemical reaction.
3. Example problems are provided to illustrate calculating molarity, empirical and molecular formulas, and amount of products formed using stoichiometric calculations and identifying the limiting reagent.
Introduction To Chemistry Power Point

This document provides an introduction to basic chemistry concepts including the structure of atoms, atomic mass, and quantities. Atoms consist of a dense positively charged nucleus made up of protons and neutrons surrounded by an electron cloud of negatively charged electrons. The number of protons determines the element. Atomic mass is measured in atomic mass units and is based on the number of protons and neutrons. Elements can have different isotopes that have the same number of protons but different numbers of neutrons, resulting in different atomic masses. Quantities in chemistry such as moles and molarity are also introduced.
ATOMS, MOLECULES AND IONS

The document discusses the atomic theory of matter and the development of atomic structure models. It describes John Dalton's atomic theory which stated that elements are composed of atoms that are unique and atoms are neither created nor destroyed in chemical reactions. The discovery of the electron by J.J. Thompson and experiments by Robert Millikan and Ernest Rutherford helped develop the modern atomic structure model of a small, dense nucleus surrounded by electrons. The document also discusses isotopes, atomic numbers, mass numbers, and how the periodic table is arranged based on atomic structure.
CH2 (1).pdf

This document provides an overview of atomic structure and bonding. It begins by reviewing John Dalton's atomic theory from 1808. It then discusses experiments that revealed subatomic particles like electrons, protons, and neutrons, leading to Rutherford's nuclear model of the atom. The document explains atomic number, mass number, and isotopes. It also introduces the periodic table and how it is arranged based on atomic structure. Finally, it distinguishes between molecular compounds formed by covalent bonds and ionic compounds formed by ionic bonds.
CHEM1 NOTES, B.pptx

This document provides information about chemistry concepts including the atomic theory, structure of the atom, atomic mass units, atomic weights, elements, compounds, and pure substances vs mixtures. Key points include:
- John Dalton formulated the atomic theory including that atoms are indivisible and differ between elements.
- Rutherford discovered the nuclear model of the atom with a small, dense nucleus and electrons in empty space around it.
- Atoms contain protons, neutrons, and electrons. Electrons are arranged in shells around the nucleus.
- Atomic mass units are used to express atomic masses rather than grams due to atoms' small size. Atomic weights are relative masses of atoms compared to carbon-12.
Atomic Theory.pptx

Rutherford's gold foil experiment involved firing alpha particles at a thin sheet of gold foil and observing their scattering. The key findings were:
- The nucleus is small and dense
- It is positively charged
- Most alpha particles passed through the foil with little deflection, but a few were greatly deflected, surprising Rutherford.
This led to the conclusion that atoms have a small, dense, positively charged nucleus surrounded by orbiting electrons. The modern atomic model consists of protons and neutrons in the nucleus surrounded by electrons in an electron cloud.
Amount of substance

This document discusses the mole concept and stoichiometry calculations. It defines key terms like the mole, molar mass, empirical and molecular formulas, and limiting reagents. It also describes how to use balanced chemical equations and molar relationships to calculate amounts of reactants and products involved in chemical reactions. Experimental procedures are provided for determining relative molecular masses and identifying unknown metals based on stoichiometric calculations.
Ch02 lecture

Here are the steps to complete the table:
1. Atomic number = number of protons
2. For neutral atoms, number of electrons = number of protons
3. Mass number = number of protons + number of neutrons
4. Atomic symbol is the element symbol
So the completed table would be:
Protons Neutrons Electrons Atomic Number Mass Number Atomic Symb
8 8 8 8 16 O
13 13 13 13 26 Fe
79 79 X 79 79 158 Au
The only unknown is the number of neutrons, which is calculated by taking the mass number and subtracting the number of protons.
Grade 9, U1-L9-Atomic structure

This document discusses the structure of atoms and their subatomic particles. It explains that atoms are made up of protons, neutrons, and electrons. Protons and neutrons are located in the nucleus at the center of the atom, while electrons surround the nucleus in energy levels. The number of protons determines the element and is known as the atomic number. The total number of protons and neutrons is the mass number. Standard atomic notation uses the mass number as a superscript on the left of the element symbol and the atomic number as a subscript.
Chapter 4 pp (1)

This document summarizes key concepts about atomic structure:
1) It describes early atomic models including Democritus' idea of indivisible atoms and Dalton's atomic theory.
2) It explains the discovery of subatomic particles (electrons, protons, neutrons) and the nuclear model of the atom with electrons orbiting a nucleus.
3) It defines important atomic properties including atomic number, mass number, isotopes, and how to calculate atomic mass.
4) It provides an overview of how the periodic table organizes elements based on these atomic properties.
Similar to Lower Six Chemistry.pptx (20)
namma_kalvi_11th_chemistry_unit_1_ppt_em_218218.pptx

namma_kalvi_11th_chemistry_unit_1_ppt_em_218218.pptx
Basic Concept of Chemistry new.pptx Wajid ullah umar

Basic Concept of Chemistry new.pptx Wajid ullah umar
Student copy of Stoichiometry sample calculations.pptx

Student copy of Stoichiometry sample calculations.pptx
Recently uploaded
The cost of acquiring information by natural selection

This is a short talk that I gave at the Banff International Research Station workshop on Modeling and Theory in Population Biology. The idea is to try to understand how the burden of natural selection relates to the amount of information that selection puts into the genome.
It's based on the first part of this research paper:
The cost of information acquisition by natural selection
Ryan Seamus McGee, Olivia Kosterlitz, Artem Kaznatcheev, Benjamin Kerr, Carl T. Bergstrom
bioRxiv 2022.07.02.498577; doi: https://doi.org/10.1101/2022.07.02.498577
Direct Seeded Rice - Climate Smart Agriculture

Direct Seeded Rice - Climate Smart AgricultureInternational Food Policy Research Institute- South Asia Office
PPT on Direct Seeded Rice presented at the three-day 'Training and Validation Workshop on Modules of Climate Smart Agriculture (CSA) Technologies in South Asia' workshop on April 22, 2024.
Compexometric titration/Chelatorphy titration/chelating titration

Classification
Metal ion ion indicators
Masking and demasking reagents
Estimation of Magnisium sulphate
Calcium gluconate
Complexometric Titration/ chelatometry titration/chelating titration, introduction, Types-
1.Direct Titration
2.Back Titration
3.Replacement Titration
4.Indirect Titration
Masking agent, Demasking agents
formation of complex
comparition between masking and demasking agents,
Indicators/Metal ion indicators/ Metallochromic indicators/pM indicators,
Visual Technique,PM indicators (metallochromic), Indicators of pH, Redox Indicators
Instrumental Techniques-Photometry
Potentiometry
Miscellaneous methods.
Complex titration with EDTA.
Randomised Optimisation Algorithms in DAPHNE

Slides from talk:
Aleš Zamuda: Randomised Optimisation Algorithms in DAPHNE .
Austrian-Slovenian HPC Meeting 2024 – ASHPC24, Seeblickhotel Grundlsee in Austria, 10–13 June 2024
https://ashpc.eu/
The binding of cosmological structures by massless topological defects

Assuming spherical symmetry and weak field, it is shown that if one solves the Poisson equation or the Einstein field
equations sourced by a topological defect, i.e. a singularity of a very specific form, the result is a localized gravitational
field capable of driving flat rotation (i.e. Keplerian circular orbits at a constant speed for all radii) of test masses on a thin
spherical shell without any underlying mass. Moreover, a large-scale structure which exploits this solution by assembling
concentrically a number of such topological defects can establish a flat stellar or galactic rotation curve, and can also deflect
light in the same manner as an equipotential (isothermal) sphere. Thus, the need for dark matter or modified gravity theory is
mitigated, at least in part.
在线办理(salfor毕业证书)索尔福德大学毕业证毕业完成信一模一样

学校原件一模一样【微信:741003700 】《(salfor毕业证书)索尔福德大学毕业证》【微信:741003700 】学位证,留信认证(真实可查,永久存档)原件一模一样纸张工艺/offer、雅思、外壳等材料/诚信可靠,可直接看成品样本,帮您解决无法毕业带来的各种难题!外壳,原版制作,诚信可靠,可直接看成品样本。行业标杆!精益求精,诚心合作,真诚制作!多年品质 ,按需精细制作,24小时接单,全套进口原装设备。十五年致力于帮助留学生解决难题,包您满意。
本公司拥有海外各大学样板无数,能完美还原。
1:1完美还原海外各大学毕业材料上的工艺:水印,阴影底纹,钢印LOGO烫金烫银,LOGO烫金烫银复合重叠。文字图案浮雕、激光镭射、紫外荧光、温感、复印防伪等防伪工艺。材料咨询办理、认证咨询办理请加学历顾问Q/微741003700
【主营项目】
一.毕业证【q微741003700】成绩单、使馆认证、教育部认证、雅思托福成绩单、学生卡等!
二.真实使馆公证(即留学回国人员证明,不成功不收费)
三.真实教育部学历学位认证(教育部存档!教育部留服网站永久可查)
四.办理各国各大学文凭(一对一专业服务,可全程监控跟踪进度)
如果您处于以下几种情况:
◇在校期间,因各种原因未能顺利毕业……拿不到官方毕业证【q/微741003700】
◇面对父母的压力,希望尽快拿到;
◇不清楚认证流程以及材料该如何准备;
◇回国时间很长,忘记办理;
◇回国马上就要找工作,办给用人单位看;
◇企事业单位必须要求办理的
◇需要报考公务员、购买免税车、落转户口
◇申请留学生创业基金
留信网认证的作用:
1:该专业认证可证明留学生真实身份
2:同时对留学生所学专业登记给予评定
3:国家专业人才认证中心颁发入库证书
4:这个认证书并且可以归档倒地方
5:凡事获得留信网入网的信息将会逐步更新到个人身份内,将在公安局网内查询个人身份证信息后,同步读取人才网入库信息
6:个人职称评审加20分
7:个人信誉贷款加10分
8:在国家人才网主办的国家网络招聘大会中纳入资料,供国家高端企业选择人才
waterlessdyeingtechnolgyusing carbon dioxide chemicalspdf

The technology uses reclaimed CO₂ as the dyeing medium in a closed loop process. When pressurized, CO₂ becomes supercritical (SC-CO₂). In this state CO₂ has a very high solvent power, allowing the dye to dissolve easily.
Sharlene Leurig - Enabling Onsite Water Use with Net Zero Water

Sharlene Leurig - Enabling Onsite Water Use with Net Zero WaterTexas Alliance of Groundwater Districts
Presented at June 6-7 Texas Alliance of Groundwater Districts Business MeetingEquivariant neural networks and representation theory

Or: Beyond linear.
Abstract: Equivariant neural networks are neural networks that incorporate symmetries. The nonlinear activation functions in these networks result in interesting nonlinear equivariant maps between simple representations, and motivate the key player of this talk: piecewise linear representation theory.
Disclaimer: No one is perfect, so please mind that there might be mistakes and typos.
dtubbenhauer@gmail.com
Corrected slides: dtubbenhauer.com/talks.html
THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...

THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...Abdul Wali Khan University Mardan,kP,Pakistan
hematic appreciation test is a psychological assessment tool used to measure an individual's appreciation and understanding of specific themes or topics. This test helps to evaluate an individual's ability to connect different ideas and concepts within a given theme, as well as their overall comprehension and interpretation skills. The results of the test can provide valuable insights into an individual's cognitive abilities, creativity, and critical thinking skillsMicronuclei test.M.sc.zoology.fisheries.

Current Ms word generated power point presentation covers major details about the micronuclei test. It's significance and assays to conduct it. It is used to detect the micronuclei formation inside the cells of nearly every multicellular organism. It's formation takes place during chromosomal sepration at metaphase.
ESA/ACT Science Coffee: Diego Blas - Gravitational wave detection with orbita...

ESA/ACT Science Coffee: Diego Blas - Gravitational wave detection with orbita...Advanced-Concepts-Team
Presentation in the Science Coffee of the Advanced Concepts Team of the European Space Agency on the 07.06.2024.
Speaker: Diego Blas (IFAE/ICREA)
Title: Gravitational wave detection with orbital motion of Moon and artificial
Abstract:
In this talk I will describe some recent ideas to find gravitational waves from supermassive black holes or of primordial origin by studying their secular effect on the orbital motion of the Moon or satellites that are laser ranged.Describing and Interpreting an Immersive Learning Case with the Immersion Cub...

Current descriptions of immersive learning cases are often difficult or impossible to compare. This is due to a myriad of different options on what details to include, which aspects are relevant, and on the descriptive approaches employed. Also, these aspects often combine very specific details with more general guidelines or indicate intents and rationales without clarifying their implementation. In this paper we provide a method to describe immersive learning cases that is structured to enable comparisons, yet flexible enough to allow researchers and practitioners to decide which aspects to include. This method leverages a taxonomy that classifies educational aspects at three levels (uses, practices, and strategies) and then utilizes two frameworks, the Immersive Learning Brain and the Immersion Cube, to enable a structured description and interpretation of immersive learning cases. The method is then demonstrated on a published immersive learning case on training for wind turbine maintenance using virtual reality. Applying the method results in a structured artifact, the Immersive Learning Case Sheet, that tags the case with its proximal uses, practices, and strategies, and refines the free text case description to ensure that matching details are included. This contribution is thus a case description method in support of future comparative research of immersive learning cases. We then discuss how the resulting description and interpretation can be leveraged to change immersion learning cases, by enriching them (considering low-effort changes or additions) or innovating (exploring more challenging avenues of transformation). The method holds significant promise to support better-grounded research in immersive learning.
Immersive Learning That Works: Research Grounding and Paths Forward

We will metaverse into the essence of immersive learning, into its three dimensions and conceptual models. This approach encompasses elements from teaching methodologies to social involvement, through organizational concerns and technologies. Challenging the perception of learning as knowledge transfer, we introduce a 'Uses, Practices & Strategies' model operationalized by the 'Immersive Learning Brain' and ‘Immersion Cube’ frameworks. This approach offers a comprehensive guide through the intricacies of immersive educational experiences and spotlighting research frontiers, along the immersion dimensions of system, narrative, and agency. Our discourse extends to stakeholders beyond the academic sphere, addressing the interests of technologists, instructional designers, and policymakers. We span various contexts, from formal education to organizational transformation to the new horizon of an AI-pervasive society. This keynote aims to unite the iLRN community in a collaborative journey towards a future where immersive learning research and practice coalesce, paving the way for innovative educational research and practice landscapes.
快速办理(UAM毕业证书)马德里自治大学毕业证学位证一模一样

学校原件一模一样【微信:741003700 】《(UAM毕业证书)马德里自治大学毕业证学位证》【微信:741003700 】学位证,留信认证(真实可查,永久存档)原件一模一样纸张工艺/offer、雅思、外壳等材料/诚信可靠,可直接看成品样本,帮您解决无法毕业带来的各种难题!外壳,原版制作,诚信可靠,可直接看成品样本。行业标杆!精益求精,诚心合作,真诚制作!多年品质 ,按需精细制作,24小时接单,全套进口原装设备。十五年致力于帮助留学生解决难题,包您满意。
本公司拥有海外各大学样板无数,能完美还原。
1:1完美还原海外各大学毕业材料上的工艺:水印,阴影底纹,钢印LOGO烫金烫银,LOGO烫金烫银复合重叠。文字图案浮雕、激光镭射、紫外荧光、温感、复印防伪等防伪工艺。材料咨询办理、认证咨询办理请加学历顾问Q/微741003700
【主营项目】
一.毕业证【q微741003700】成绩单、使馆认证、教育部认证、雅思托福成绩单、学生卡等!
二.真实使馆公证(即留学回国人员证明,不成功不收费)
三.真实教育部学历学位认证(教育部存档!教育部留服网站永久可查)
四.办理各国各大学文凭(一对一专业服务,可全程监控跟踪进度)
如果您处于以下几种情况:
◇在校期间,因各种原因未能顺利毕业……拿不到官方毕业证【q/微741003700】
◇面对父母的压力,希望尽快拿到;
◇不清楚认证流程以及材料该如何准备;
◇回国时间很长,忘记办理;
◇回国马上就要找工作,办给用人单位看;
◇企事业单位必须要求办理的
◇需要报考公务员、购买免税车、落转户口
◇申请留学生创业基金
留信网认证的作用:
1:该专业认证可证明留学生真实身份
2:同时对留学生所学专业登记给予评定
3:国家专业人才认证中心颁发入库证书
4:这个认证书并且可以归档倒地方
5:凡事获得留信网入网的信息将会逐步更新到个人身份内,将在公安局网内查询个人身份证信息后,同步读取人才网入库信息
6:个人职称评审加20分
7:个人信誉贷款加10分
8:在国家人才网主办的国家网络招聘大会中纳入资料,供国家高端企业选择人才
SAR of Medicinal Chemistry 1st by dk.pdf

In this presentation include the prototype drug SAR on thus or with their examples .
Syllabus of Second Year B. Pharmacy
2019 PATTERN.
Recently uploaded (20)
aziz sancar nobel prize winner: from mardin to nobel

aziz sancar nobel prize winner: from mardin to nobel
The cost of acquiring information by natural selection

The cost of acquiring information by natural selection
Compexometric titration/Chelatorphy titration/chelating titration

Compexometric titration/Chelatorphy titration/chelating titration
The binding of cosmological structures by massless topological defects

The binding of cosmological structures by massless topological defects
waterlessdyeingtechnolgyusing carbon dioxide chemicalspdf

waterlessdyeingtechnolgyusing carbon dioxide chemicalspdf
Sharlene Leurig - Enabling Onsite Water Use with Net Zero Water

Sharlene Leurig - Enabling Onsite Water Use with Net Zero Water
Equivariant neural networks and representation theory

Equivariant neural networks and representation theory
THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...

THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...
ESA/ACT Science Coffee: Diego Blas - Gravitational wave detection with orbita...

ESA/ACT Science Coffee: Diego Blas - Gravitational wave detection with orbita...
Describing and Interpreting an Immersive Learning Case with the Immersion Cub...

Describing and Interpreting an Immersive Learning Case with the Immersion Cub...
Immersive Learning That Works: Research Grounding and Paths Forward

Immersive Learning That Works: Research Grounding and Paths Forward
Lower Six Chemistry.pptx
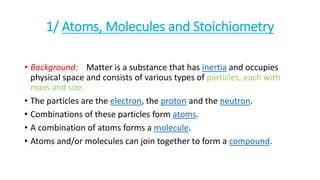
- 1. 1/ Atoms, Molecules and Stoichiometry • Background: Matter is a substance that has inertia and occupies physical space and consists of various types of particles, each with mass and size. • The particles are the electron, the proton and the neutron. • Combinations of these particles form atoms. • A combination of atoms forms a molecule. • Atoms and/or molecules can join together to form a compound.
- 2. Chemical reactions consume and produce materials knowledge of relative atomic and molecular masses. Stoichiometry: calculate the quantities of these materials e.g titrations enable the determination of the concentration of a sample
- 3. 1.1/ Relative Masses of Atoms and Molecules • atomic masses are based on 12C as the standard and 12C has a mass of 12 atomic mass units (a.m.u). • All other atoms are given masses relative to the 12C standard. • masses of atoms are compared using an instrument called a mass spectrometer.
- 4. The instrument operates as follows: • Atoms or molecules are passed into a beam of electrons moving at high speed • electrons convert the atoms or molecules being analysed into positively charged ions • The positive ions pass through an electric field which accelerates them towards a magnetic field. • Accelerating ions create their own magnetic field n interaction between the two magnetic fields results in the ion being deflected and the amount of deflection for each ion depends on its mass. • Comparison of the areas where the ions hit the detector plate enables the accurate determination of their relative masses.
- 5. • Example: When the masses of 12C and 13C are compared in a mass spectrometer their ratio is found to be:
- 6. 1.1.1/ Relative atomic masses for elements with isotopes • though carbon -12 was assigned a mass of exactly 12amu, its mass number as shown in the periodic table is 12.01 or is not 12. • since carbon is found in nature as a mixture of isotopes 12C, 13C and 14C, having different numbers of neutrons but the same number of protons. The atomic mass of 12.01 is actually an average value • Which is calculated by taking into account the relative abundances of the three isotopes. • Natural carbon consists of 98.89% of 12C, 1.11% of 13C and the amount 14C is negligible • The relative atomic mass is obtained as follows: • 98.89% of 12 amu + 1.11% of 13.003 = (0.9889 x 12 amu) + (0.011 x 13.003 amu) = 12.01 a.m.u • 12.01 is the atomic weight of carbon.
- 7. 1.1.2/ Relative atomic and molecular masses from mass spectra • The mass spectrometer is also used for determining the isotopes of a natural element. • When a sample of a naturally occurring element is analysed in a mass spectrometer, a mass spectrum is obtained. • a plot of the number of atoms against mass number. • If the mass spectrum shows a number of peaks, then they are due to the isotopes of the element. • spectrum enables the determination of the masses of each isotope and their relative abundances.
- 8. • Below is a spectrum of natural neon: • Therefore natural Neon has three • isotopes: 20Ne, 21Ne and 22Ne. • their relative abundances are 91%, 0.2% and 8.8% respectively. • The relative atomic mass of Neon is : • (0.91x20) + (0.002x 21) + (0.088 x 22) = 20.18
- 9. 1.1.3/ Relative Molecular Mass • mass spectrum of a compound shows a variety of peaks that correspond to the various fragments of the molecule. • The relative mass of the heaviest particle shown on a mass spectrum is that of the unfragmented particle with a positive charge. • This mass is taken as the relative molecular mass of the compound, the sum of all the atomic masses of the elements making up the molecule.
- 10. • Examples:
- 11. 1.1.4/ Empirical and molecular formulae of compounds • the simplest whole number ratio of the different types of atoms that make up a compound whereas the molecular formula represents the exact formula. • Two methods can be used to determine the formula of a compound. These involve the use of composition by mass data and combustion data. A weighed sample is either decomposed into its constituent elements (mass spectrometry) or it is reacted with oxygen to produce CO2, H2O and N2.
- 12. • E.g: An organic substance is composed of carbon, hydrogen and nitrogen. When 0.1156 grams of the compound was burnt in oxygen, 0.1638g of carbon dioxide CO2 and 0.1676g of Water H2O were collected. Given that the molar mass of the organic compound is 62.12 deduce its empirical and molecular formulae. • Step 1: Determine the number of atoms (in moles) of each element present • the fraction of carbon in terms of mass present in CO2 is = (atomic mass of carbon / molar mass of CO2) • Step 2: Determine the mass of elements in original sample
- 13. Step 3: Determine the moles of elements in original sample Step 4: Determine the ratios of elements in original sample