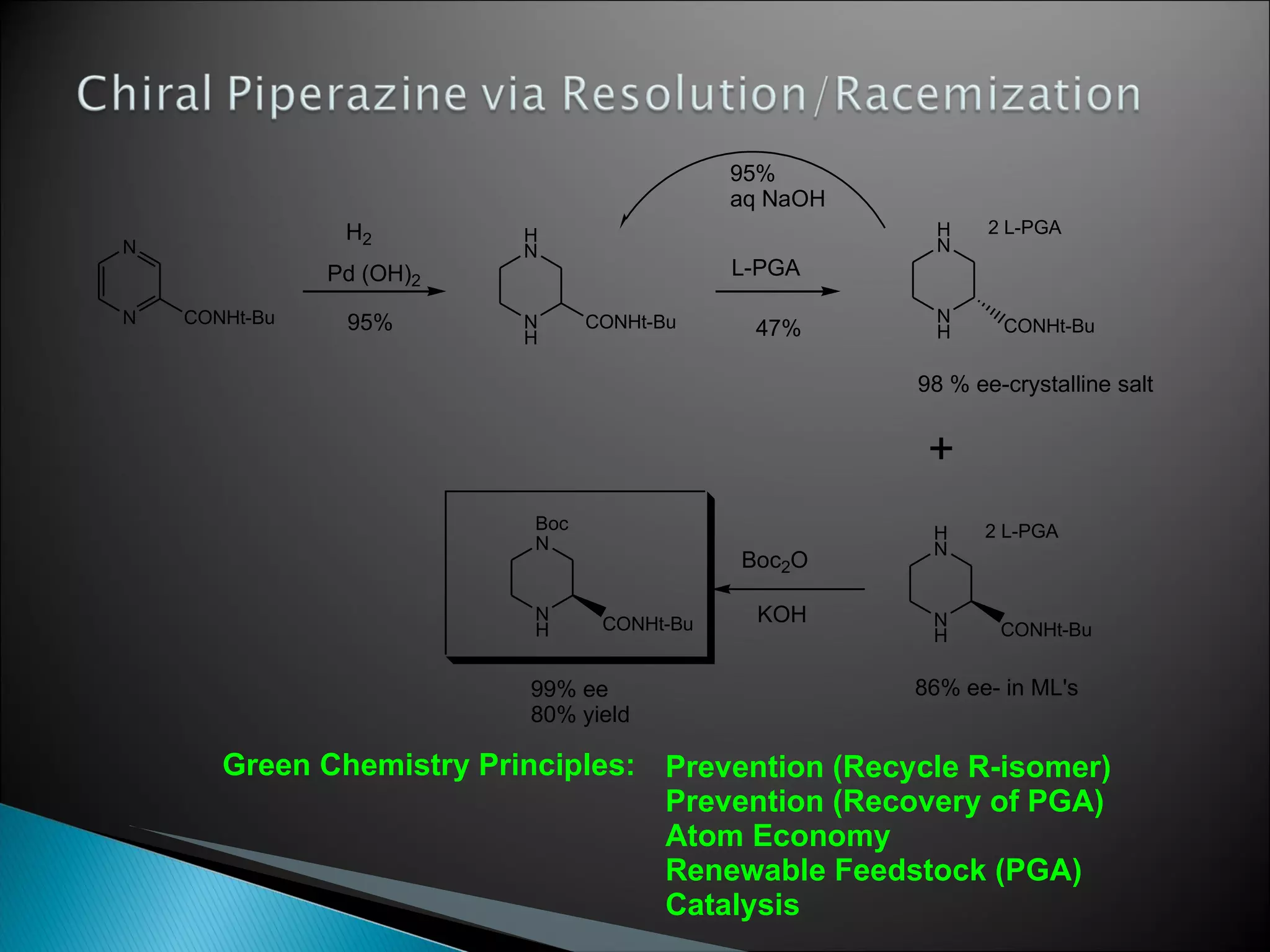
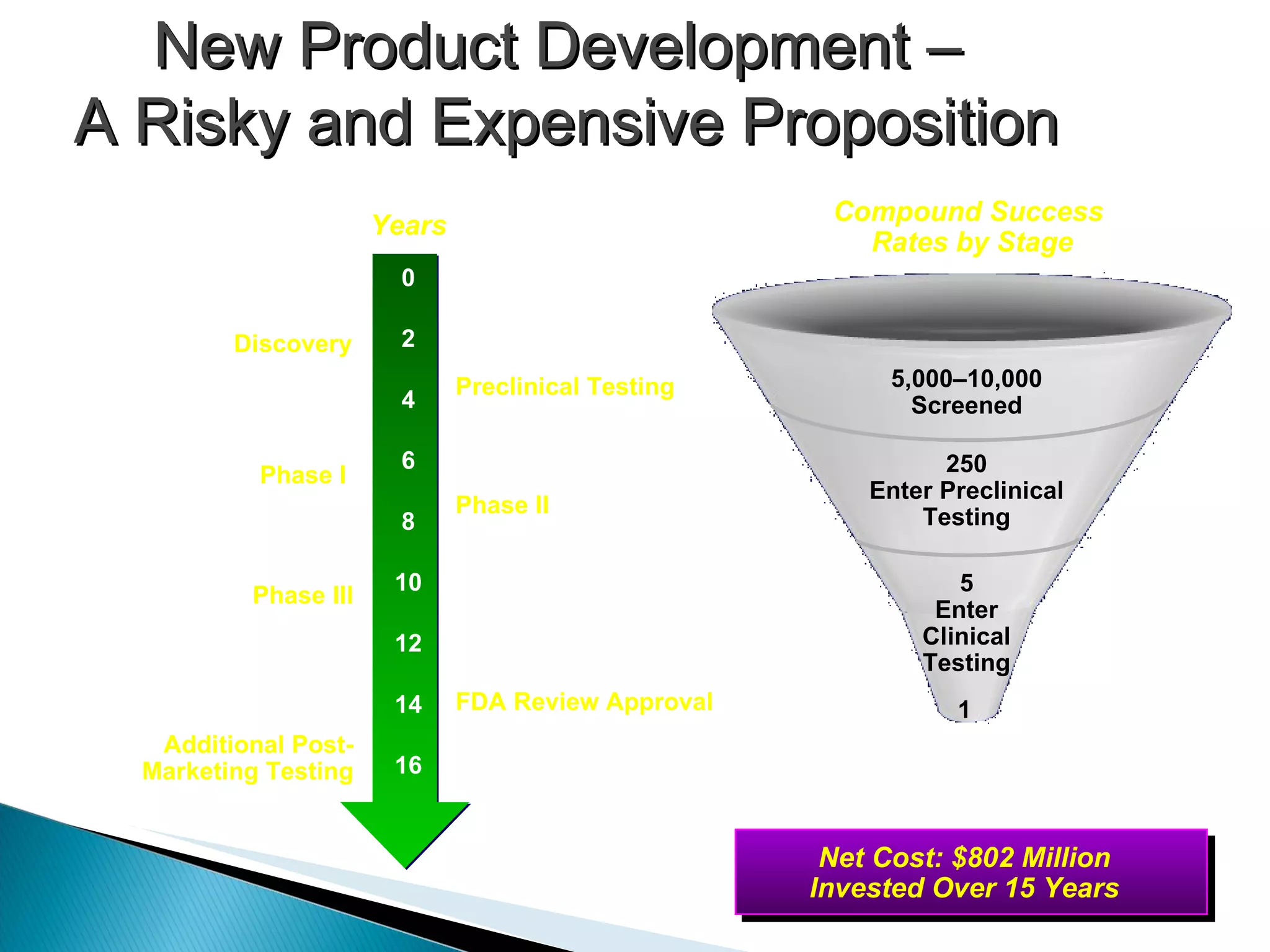
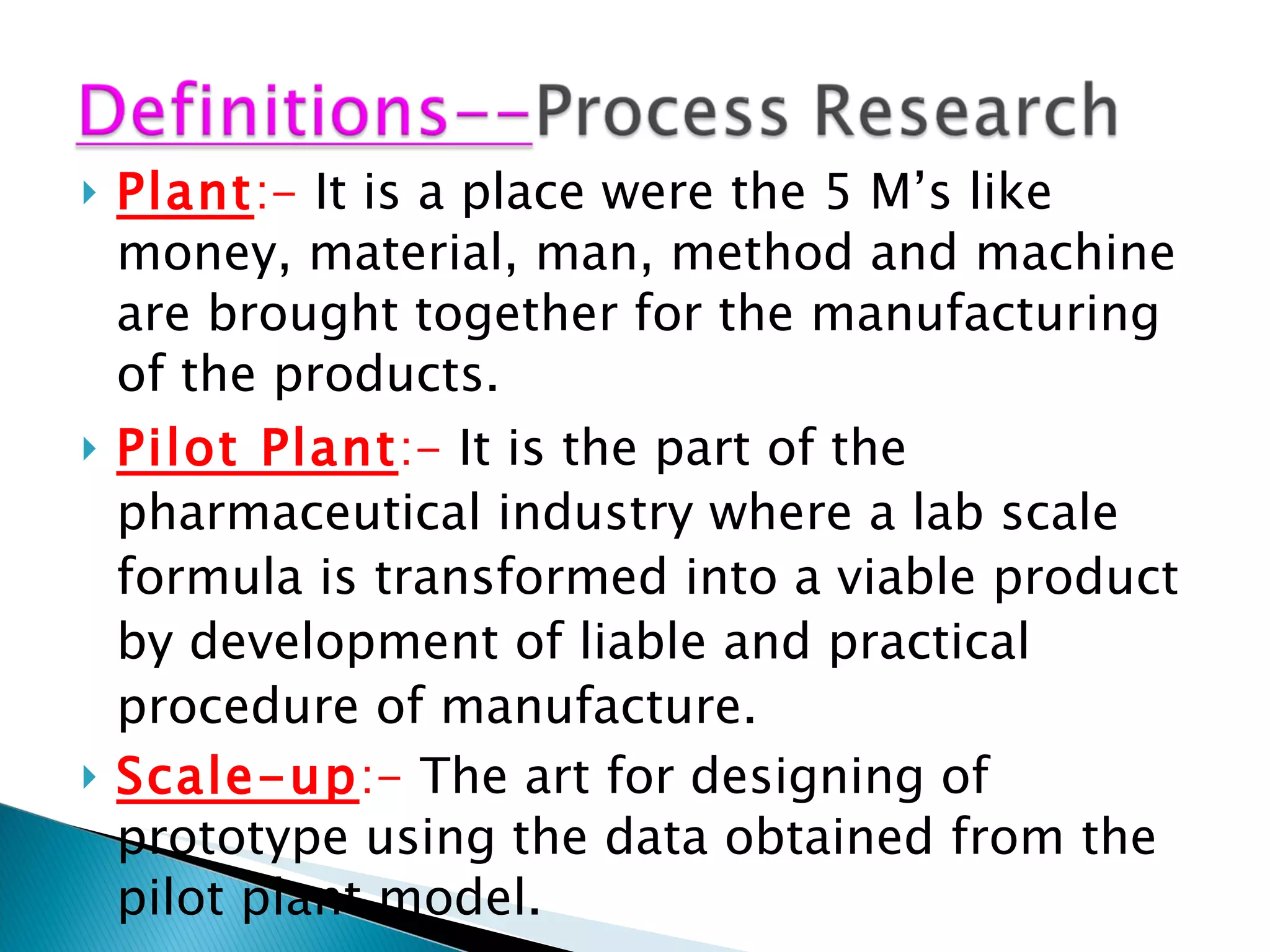
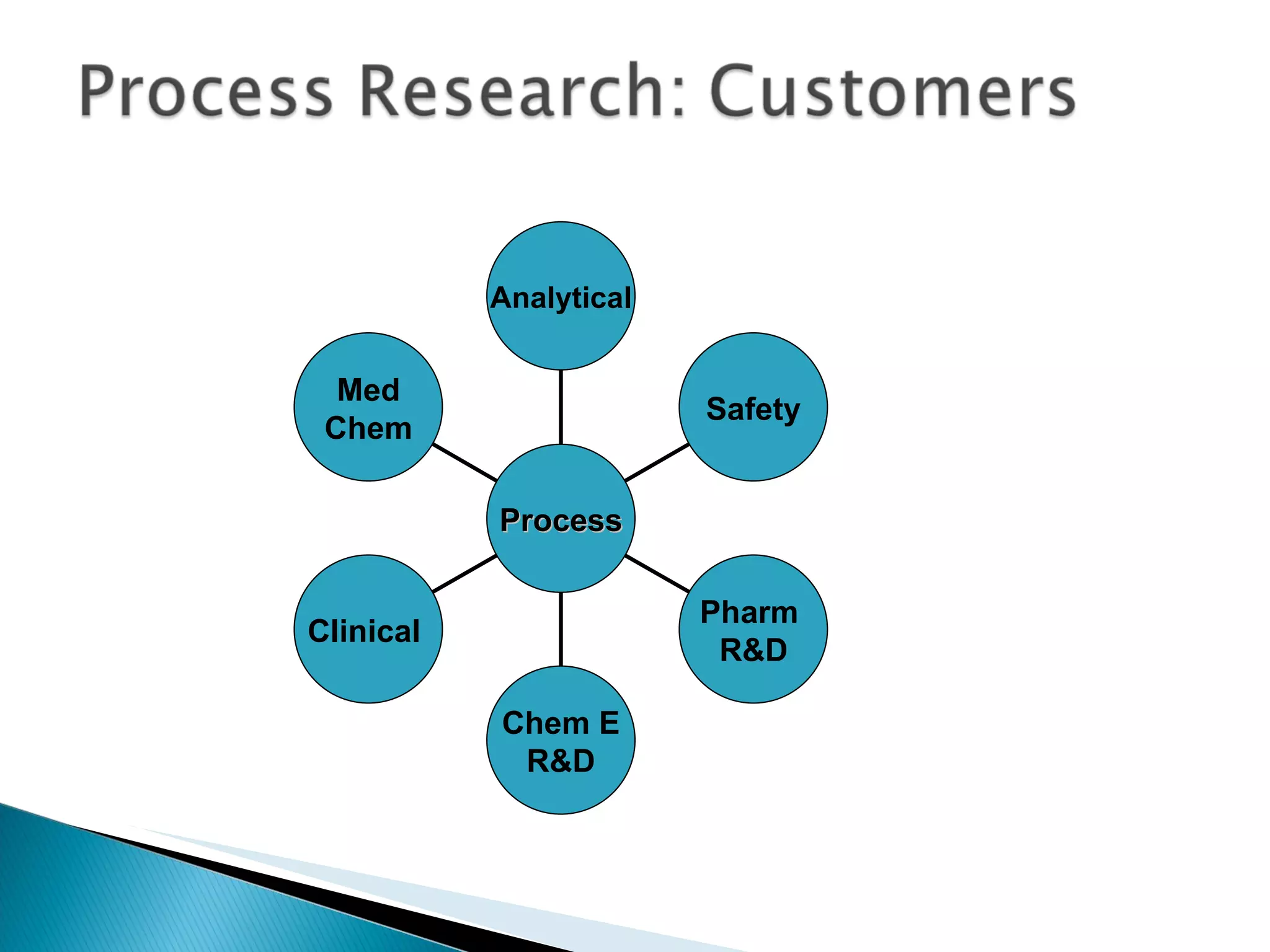
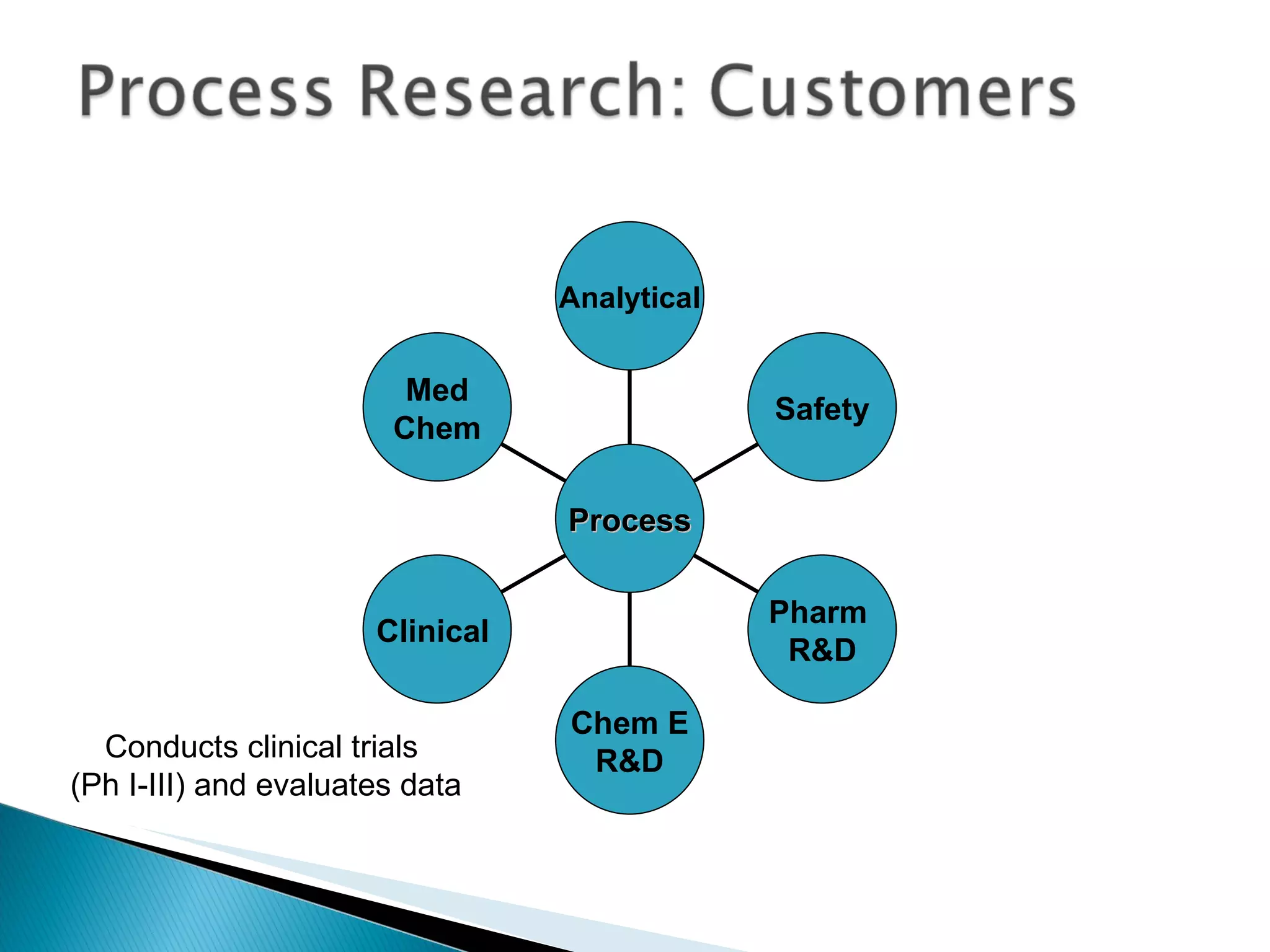
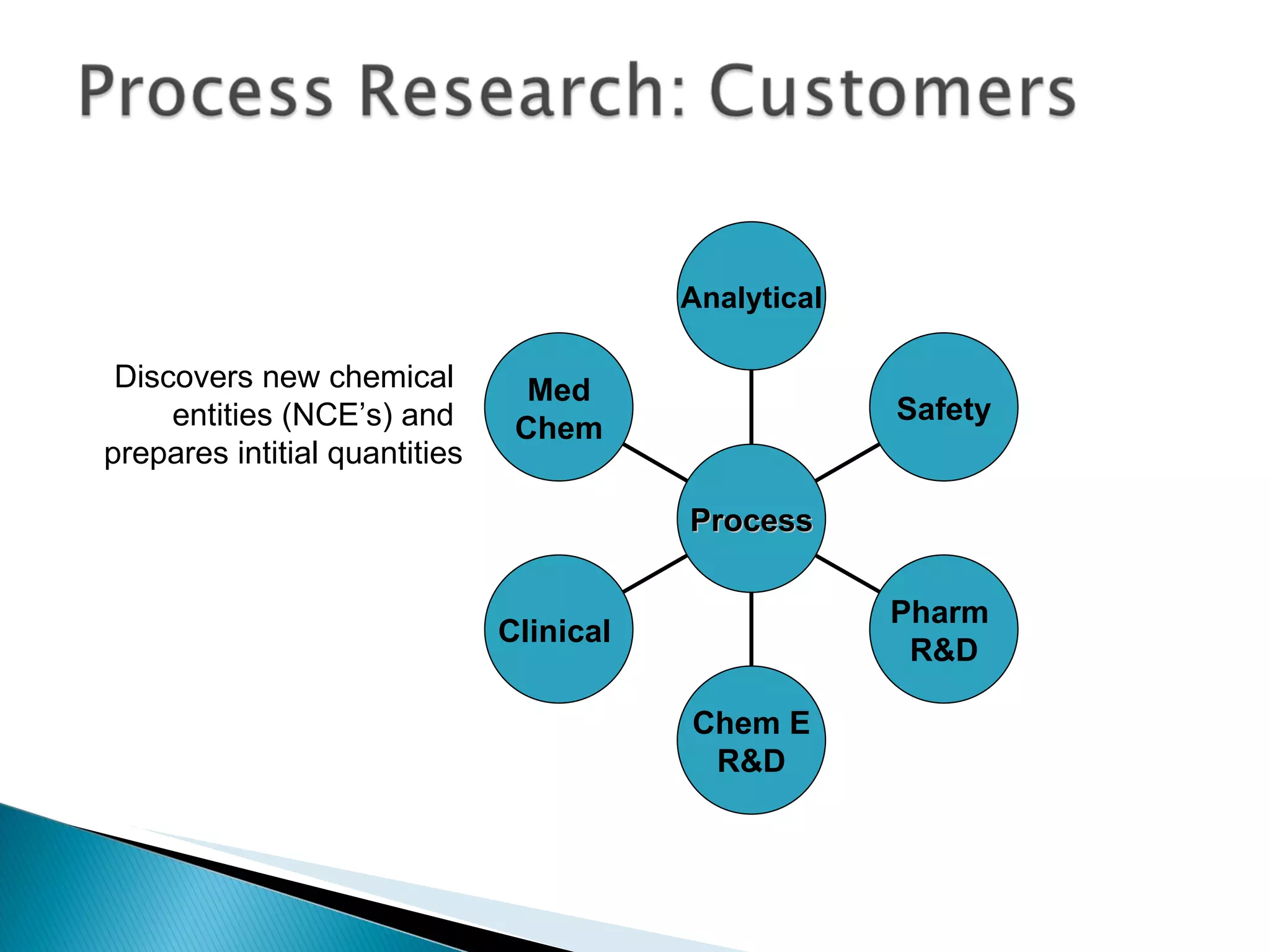
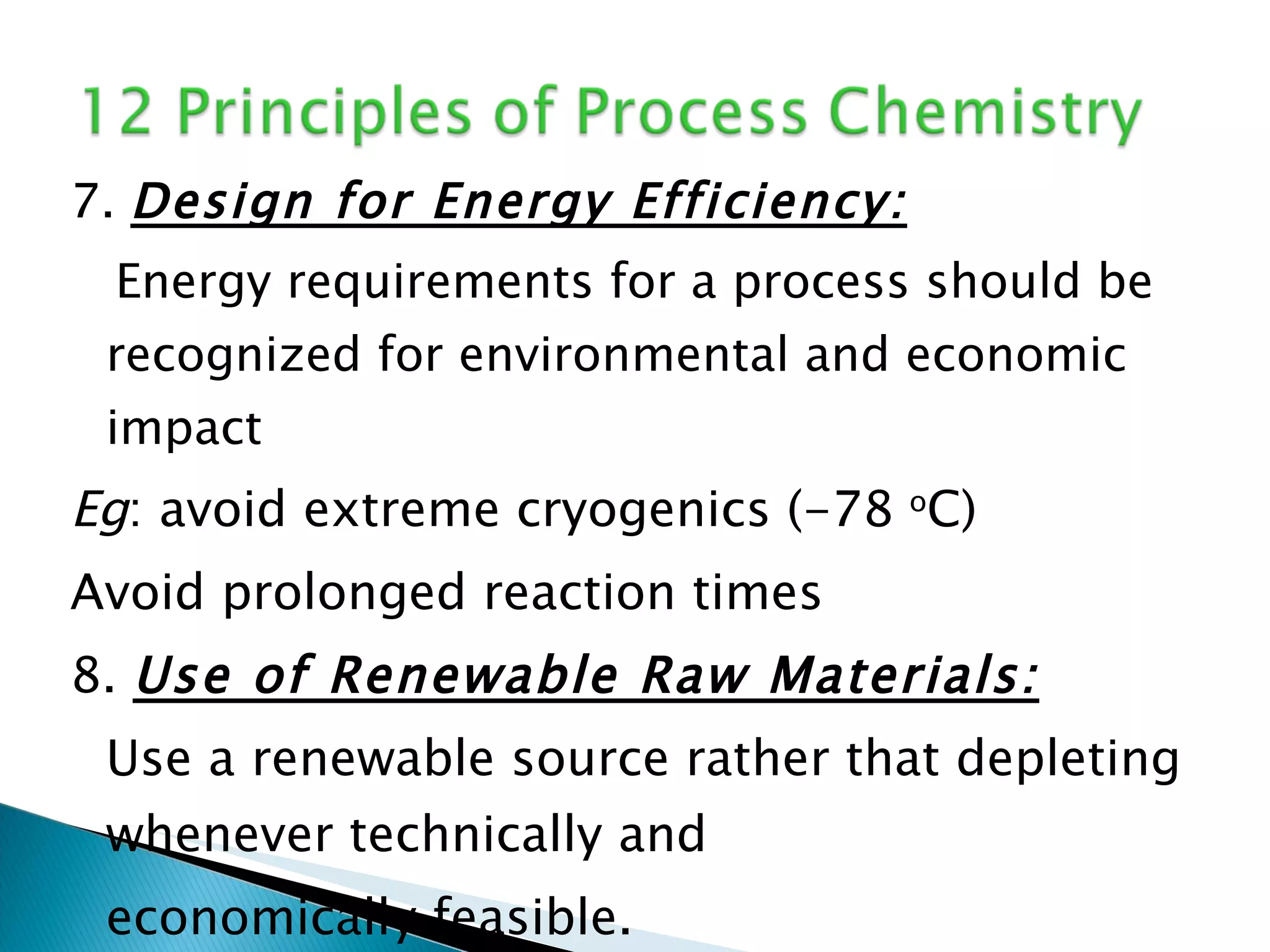
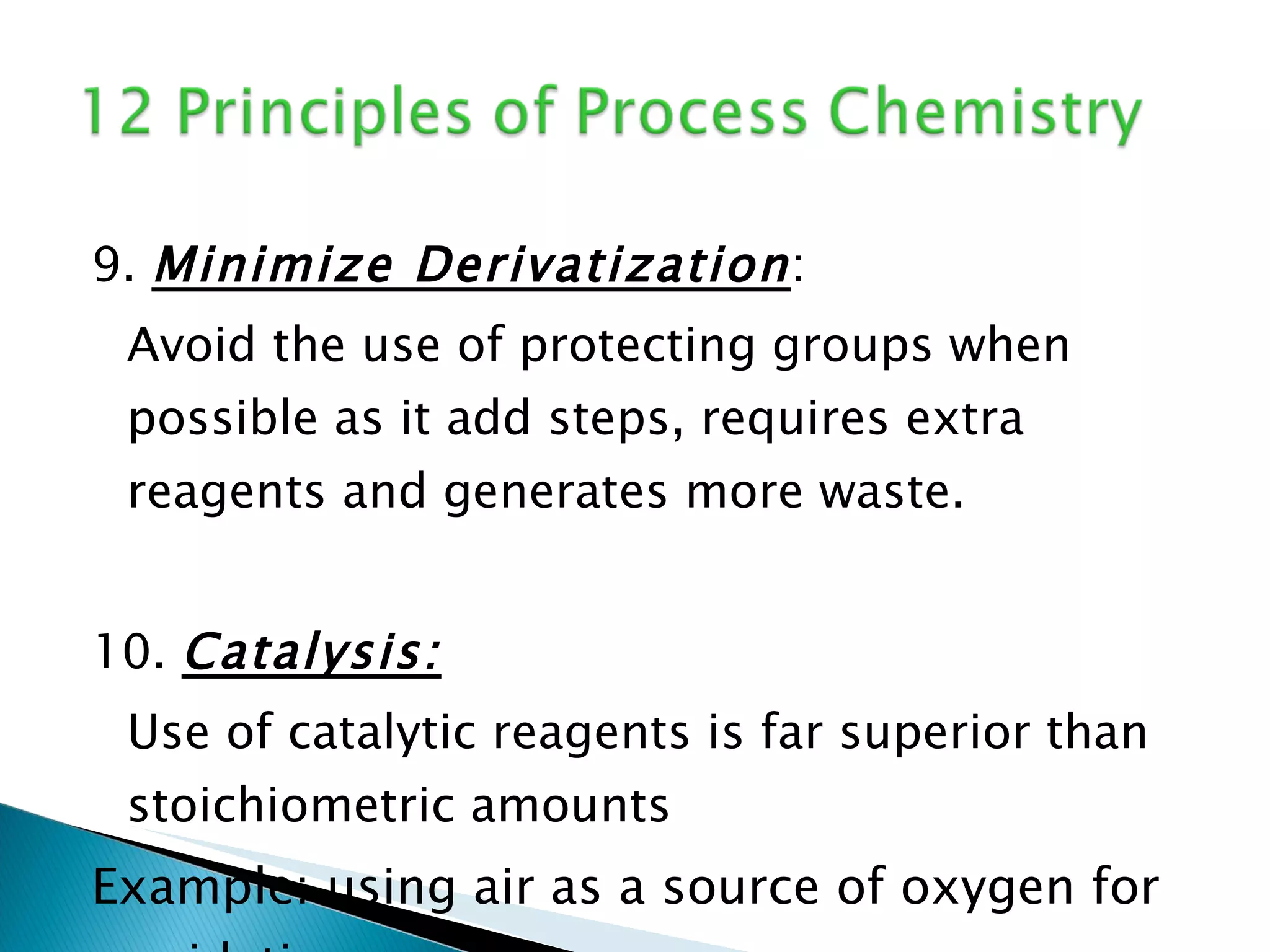
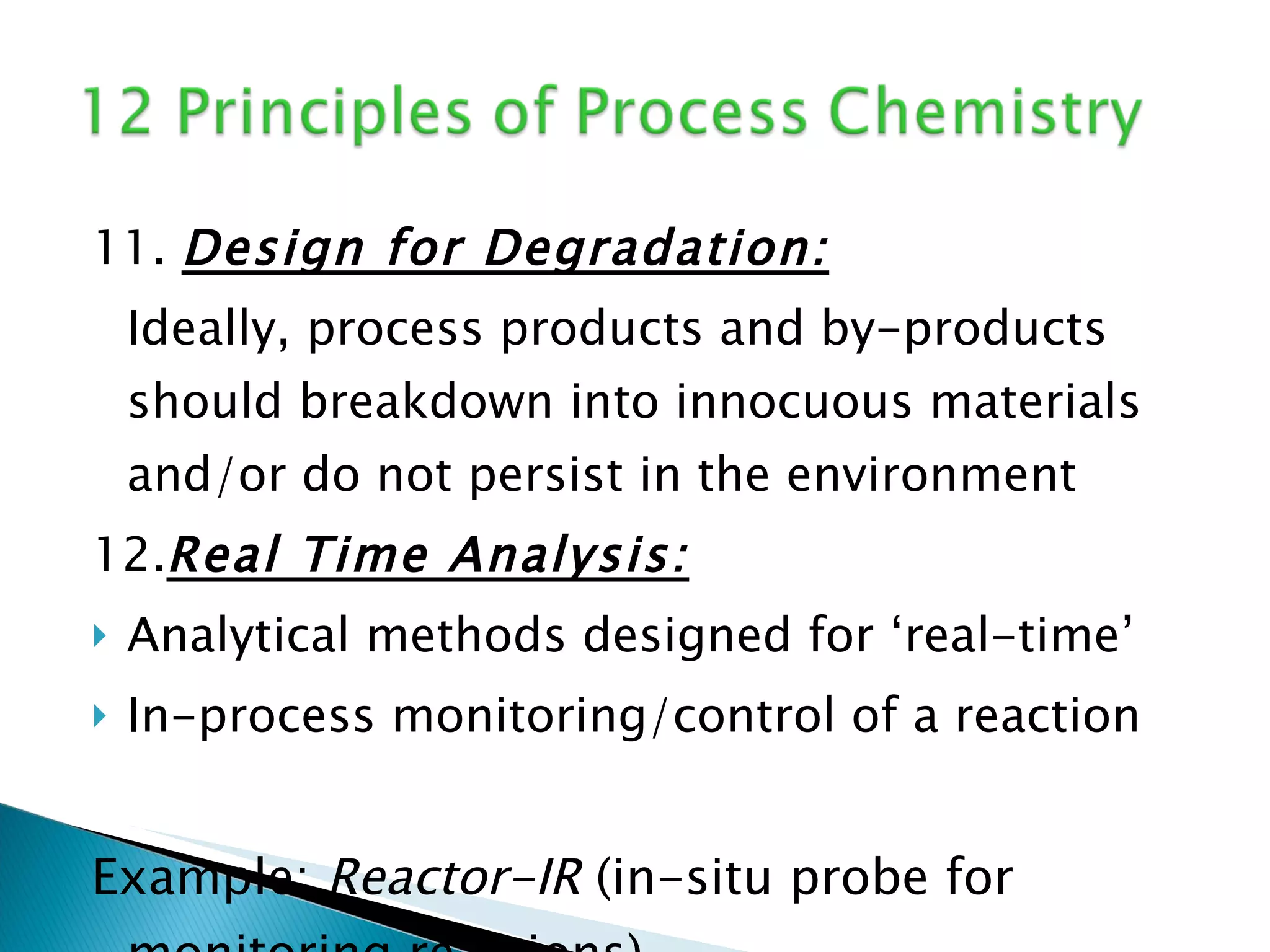
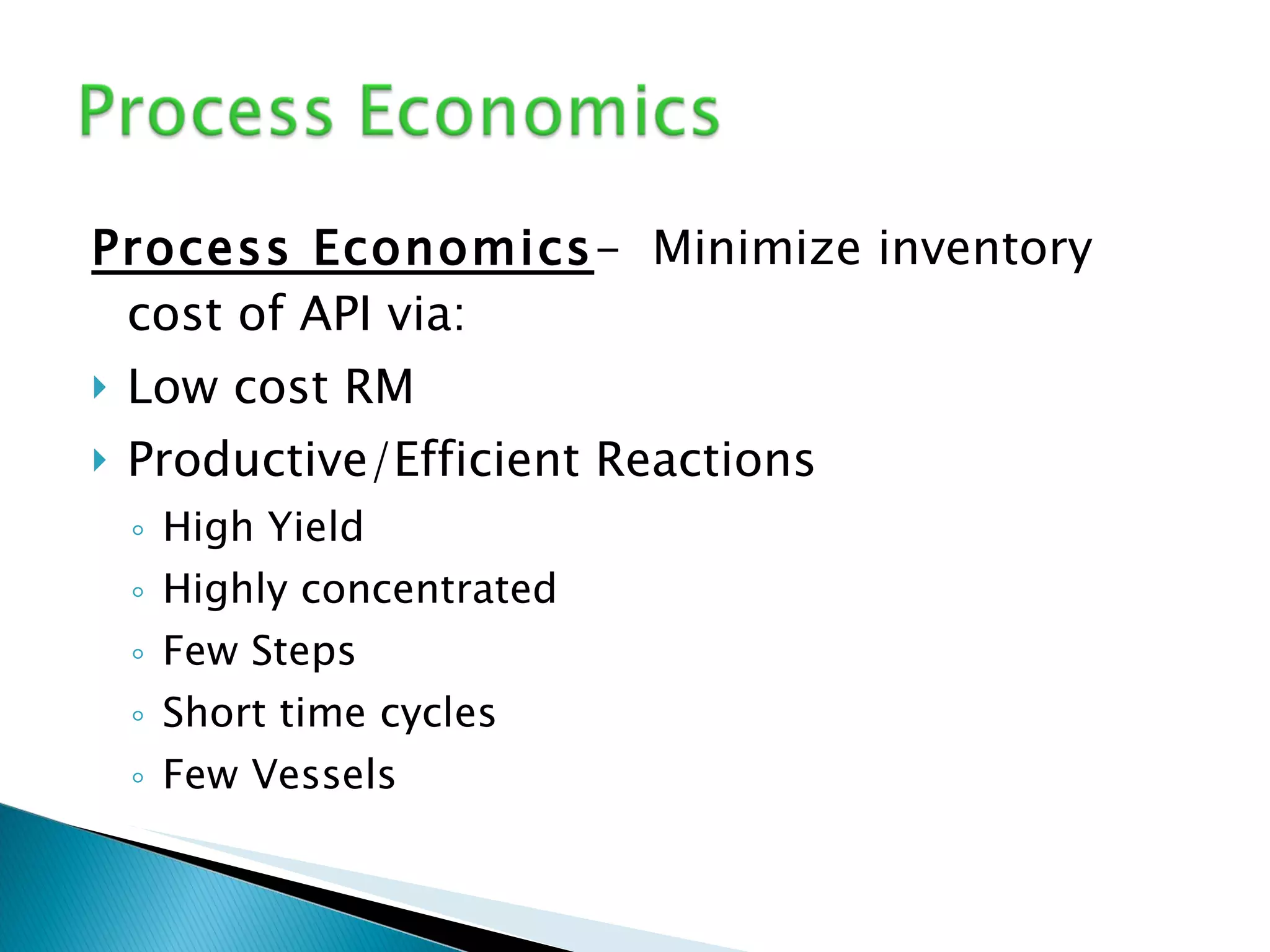
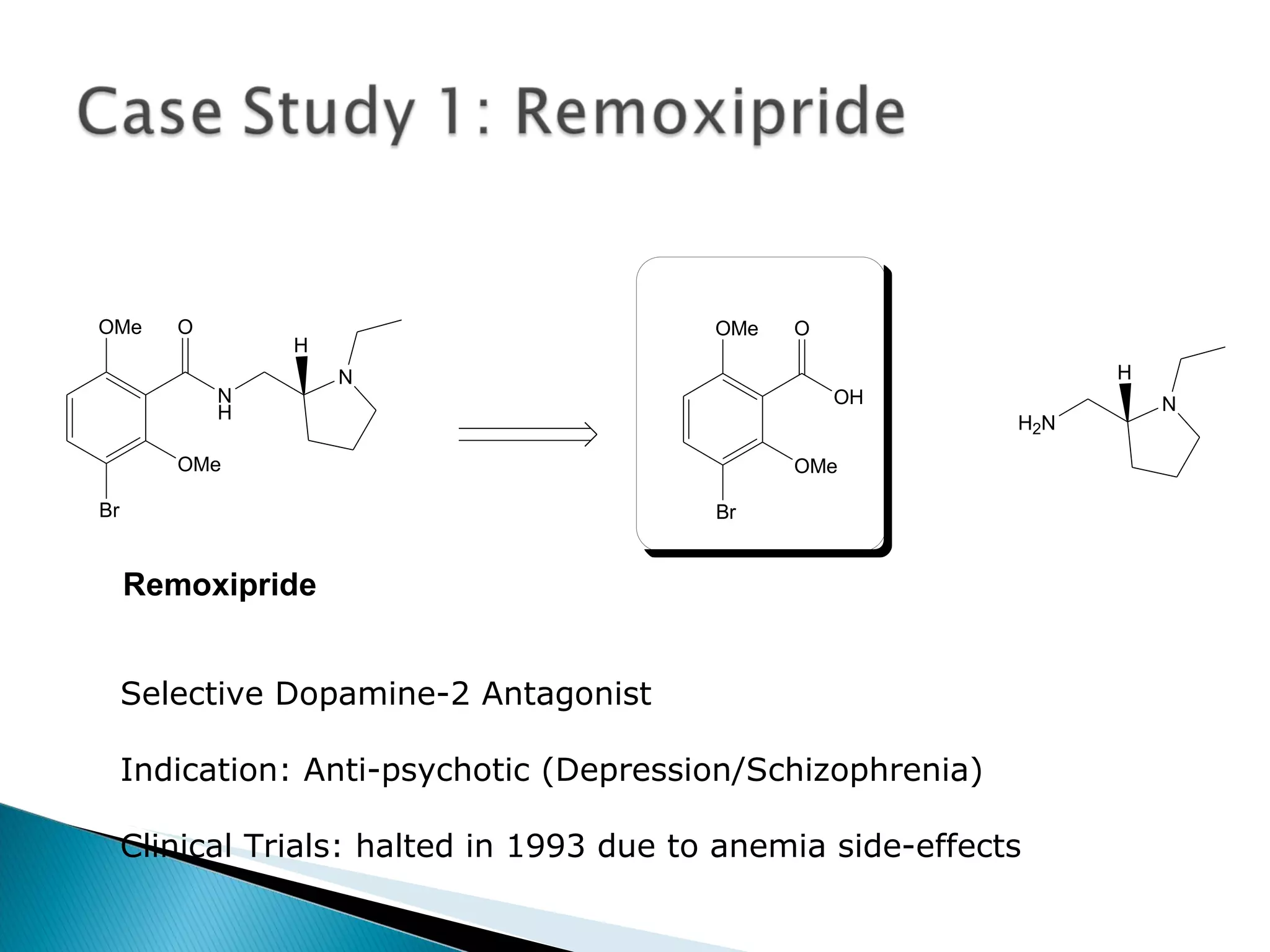
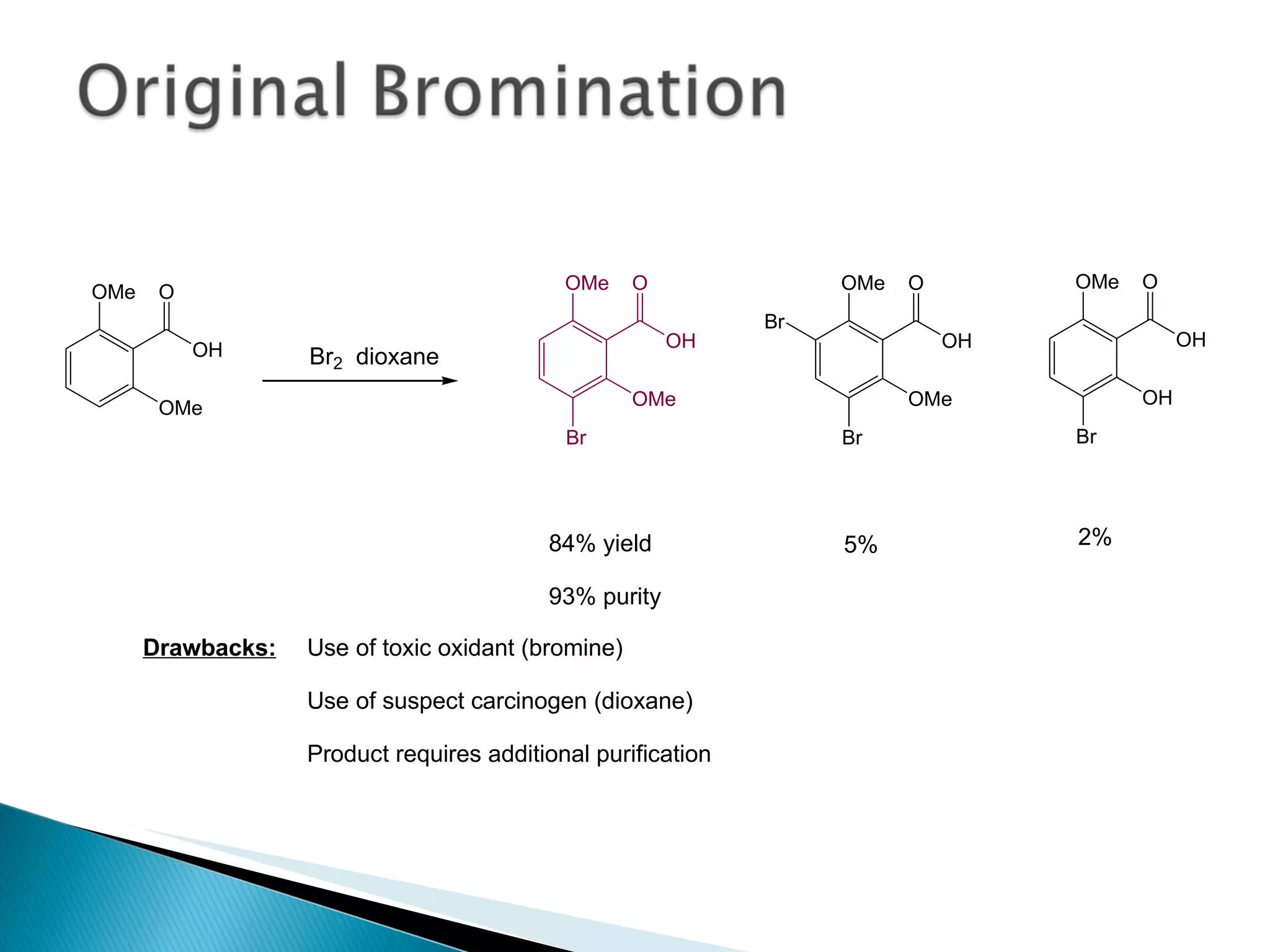
The document presents an overview of process research and development in the pharmaceutical industry, detailing its 12 principles, objectives, and stages from preclinical testing to manufacturing. Key aspects include the role of various personnel, GMP considerations, and economic challenges, along with case studies such as remoxipride and chiral piperazine. It emphasizes the importance of process innovation and outlines best practices for efficient and environmentally responsible chemical synthesis.











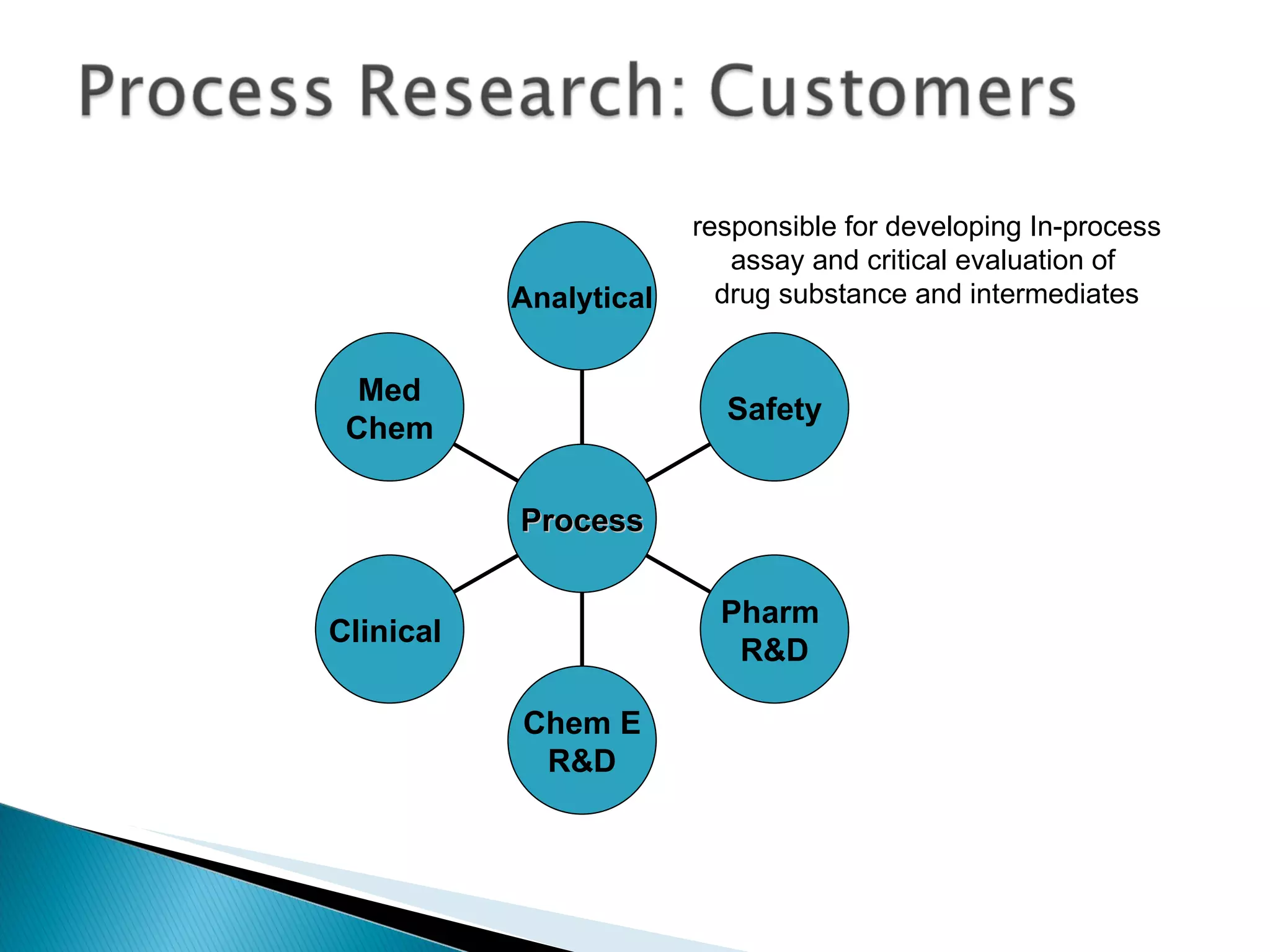
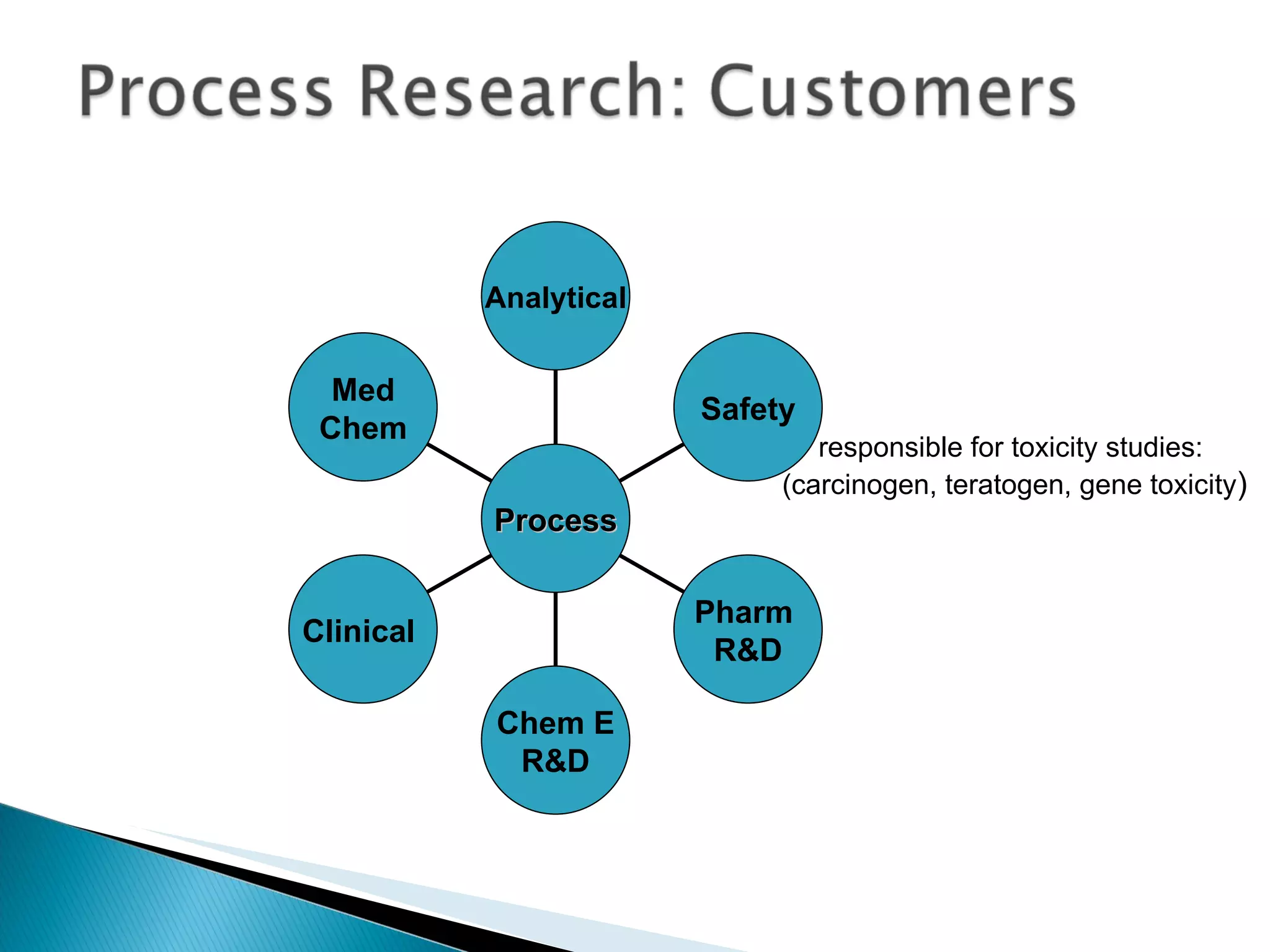
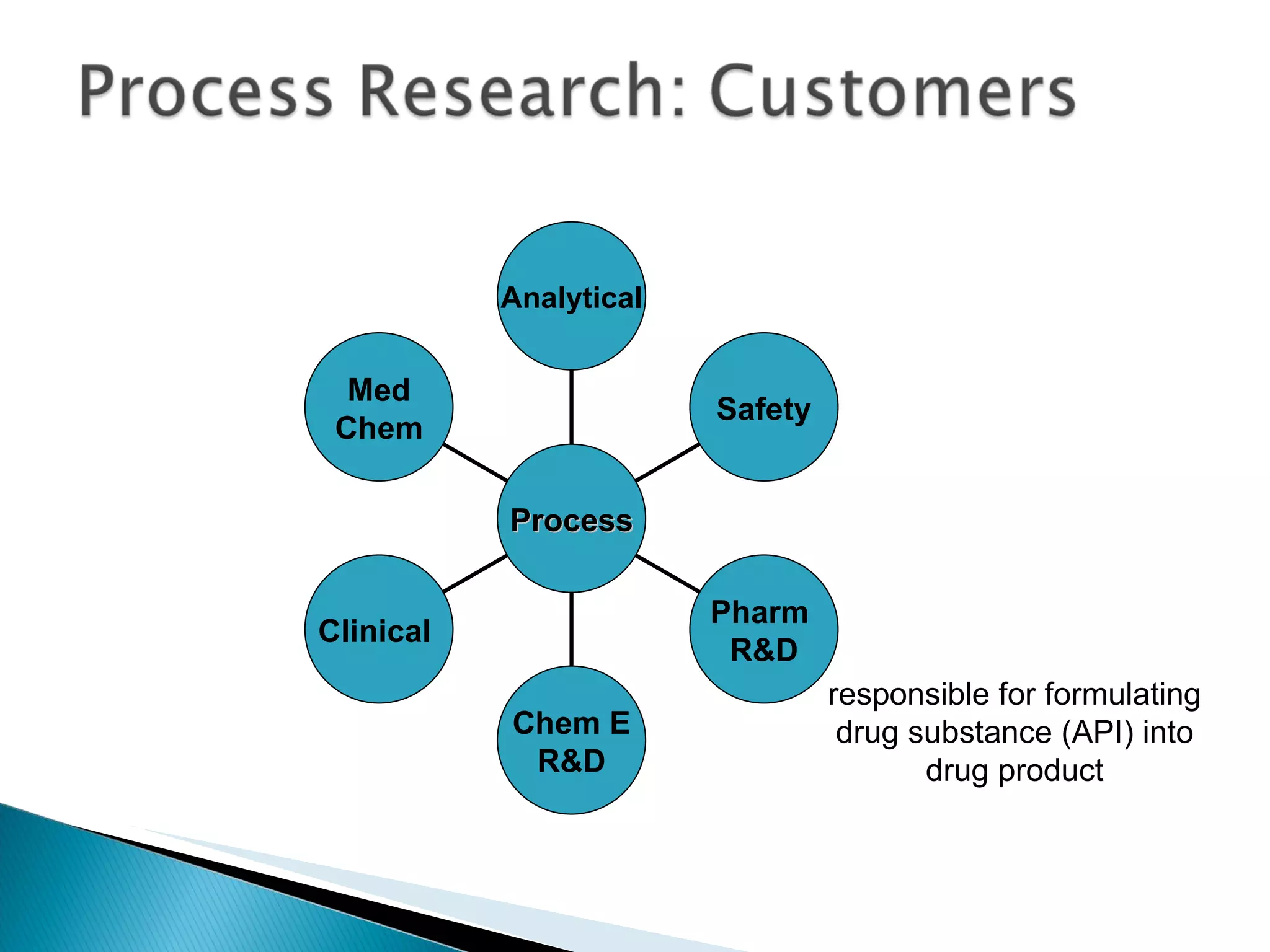
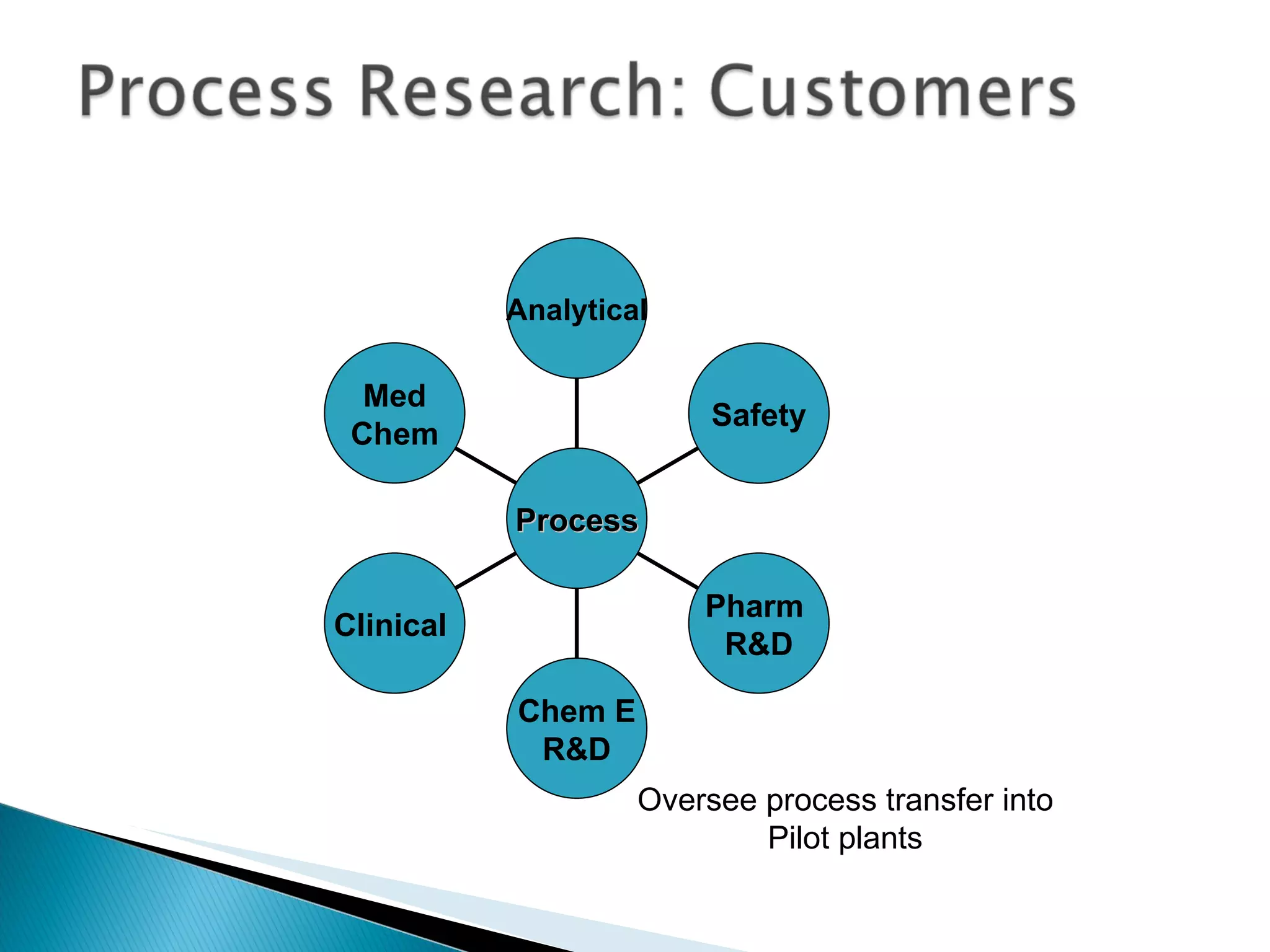




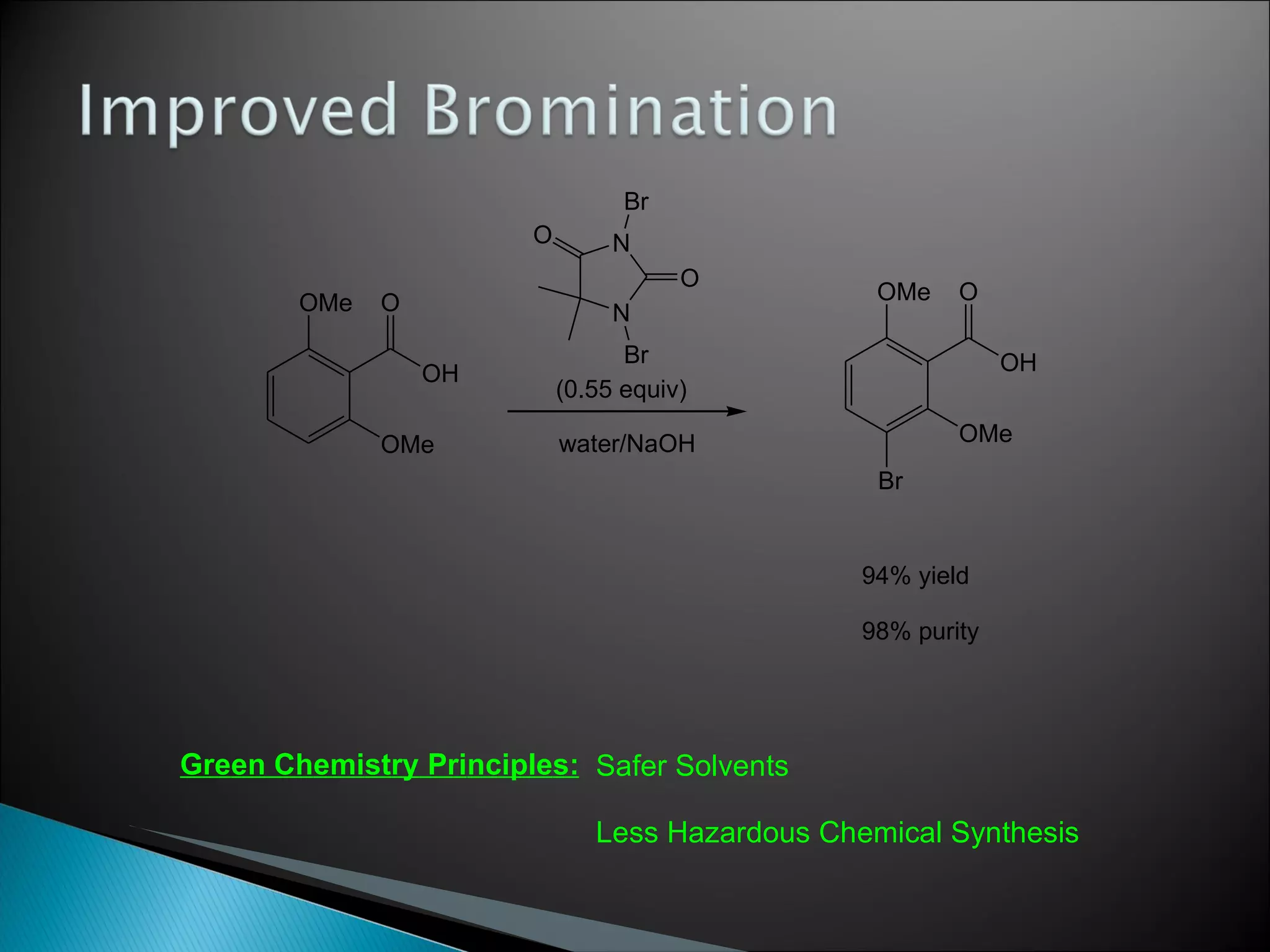
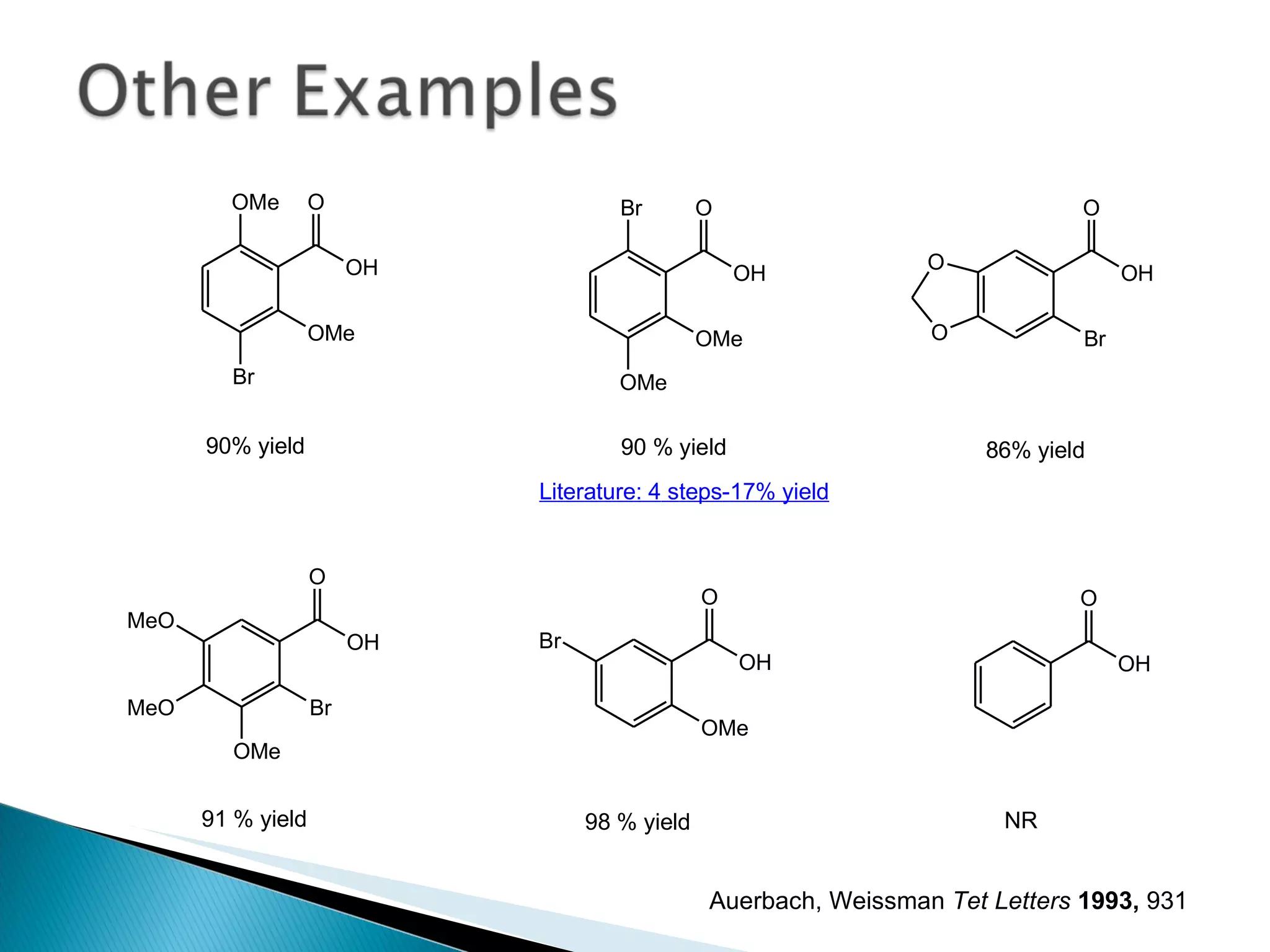
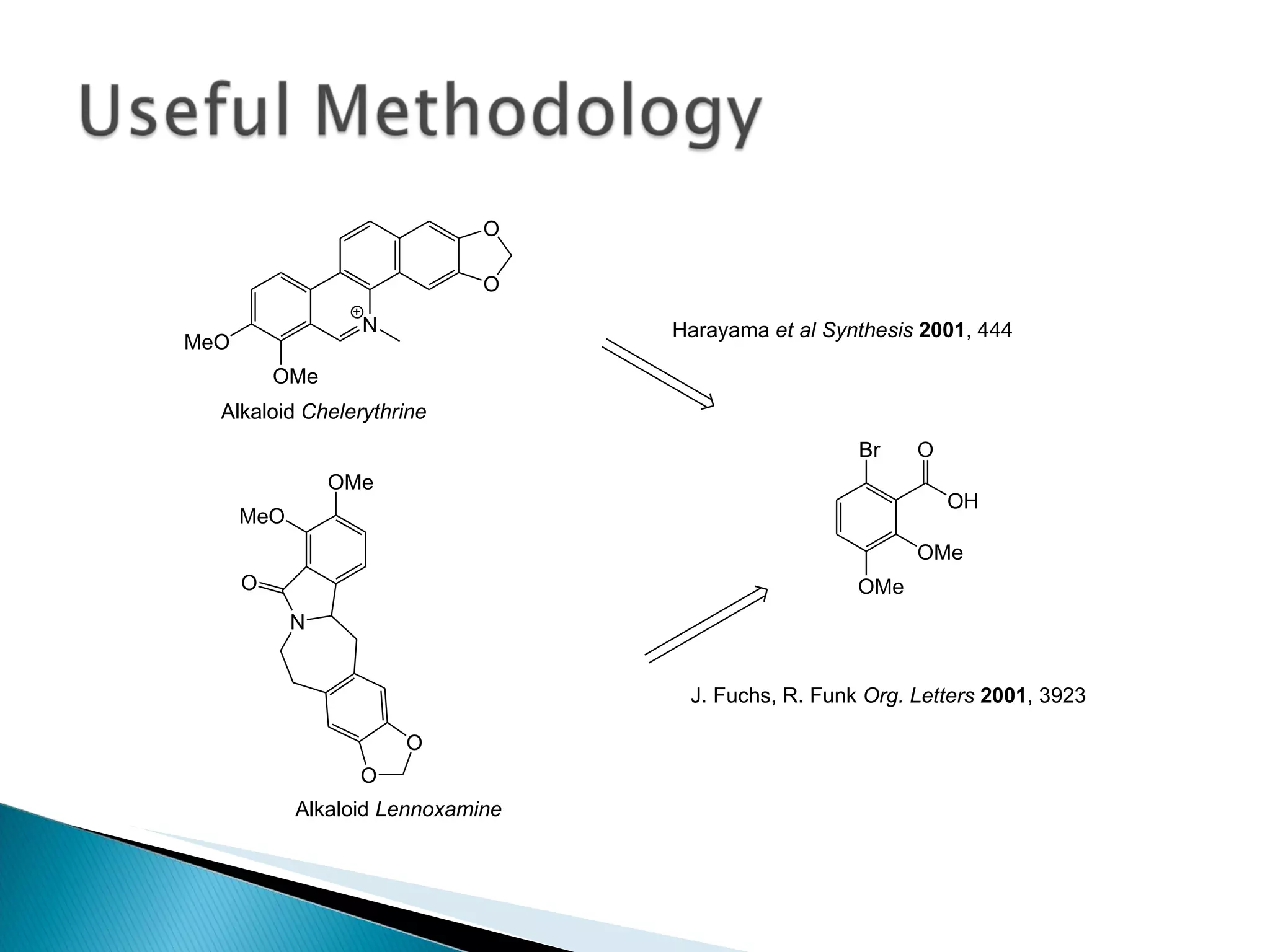
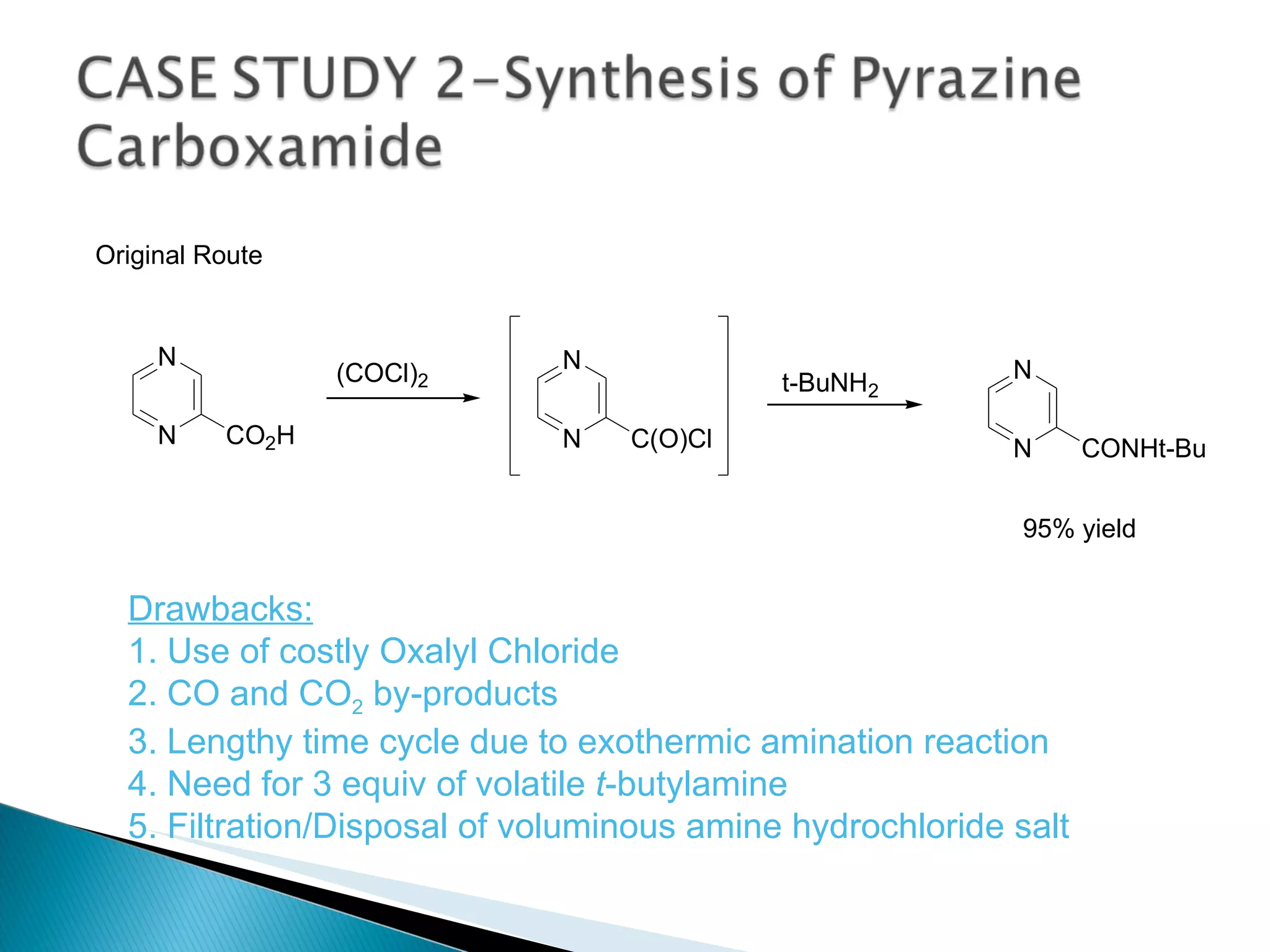
![A: 179/[124+127+73+73] = 45 % B: 179/[105 + 98 +74 +18] = 61%](https://image.slidesharecdn.com/processresearch-overview-111229025639-phpapp01/75/Process-research-overview-36-2048.jpg)