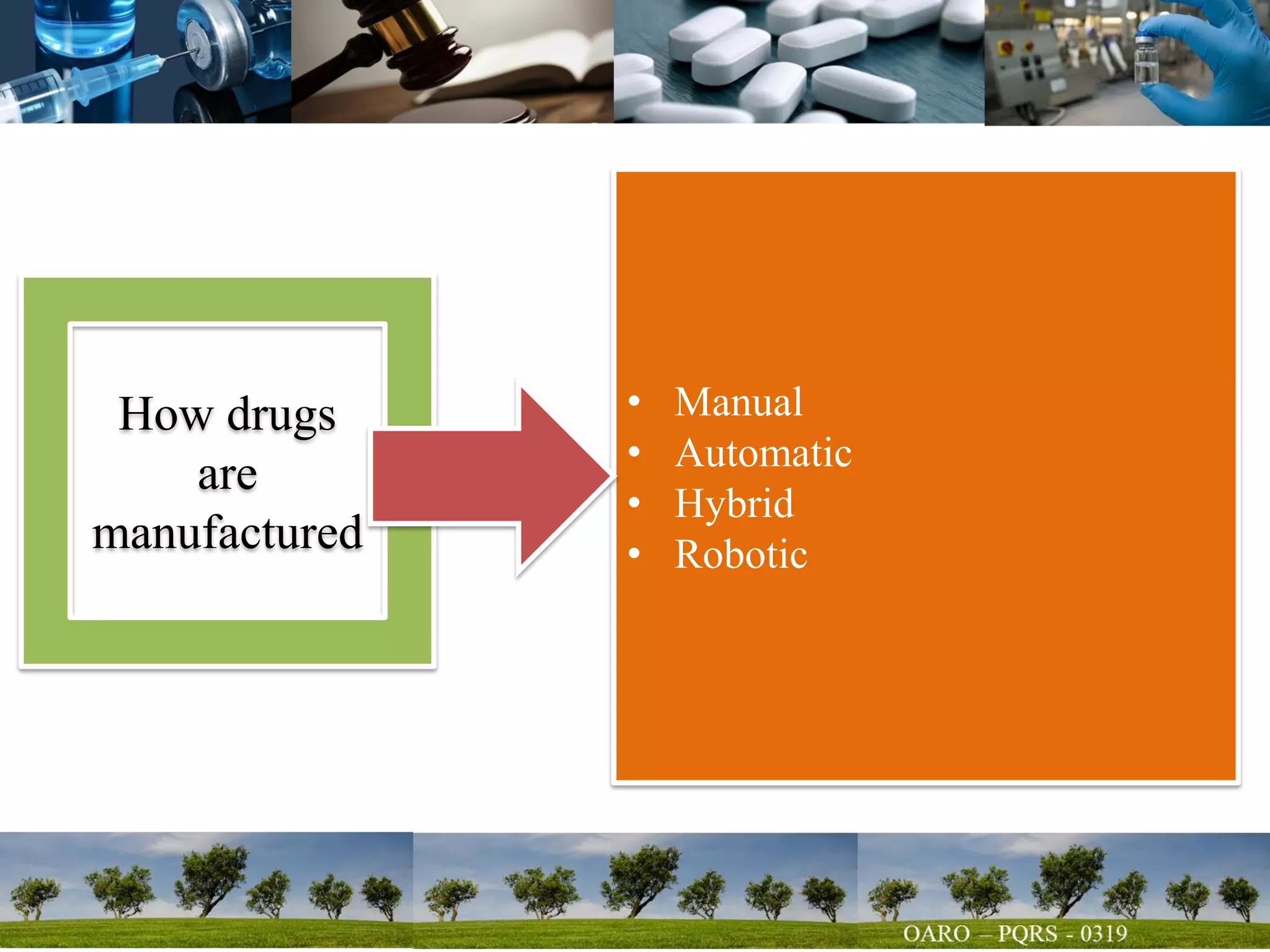
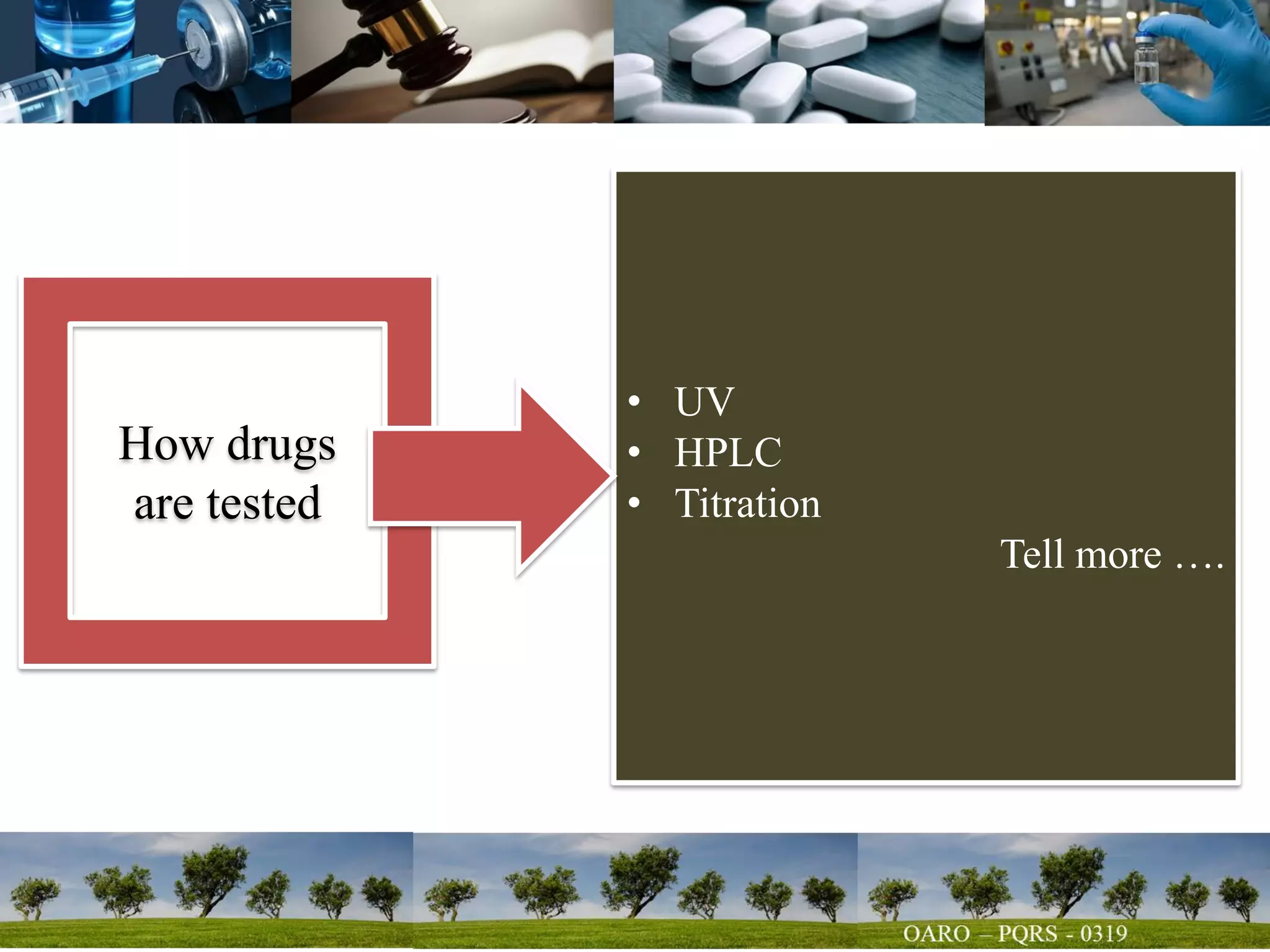
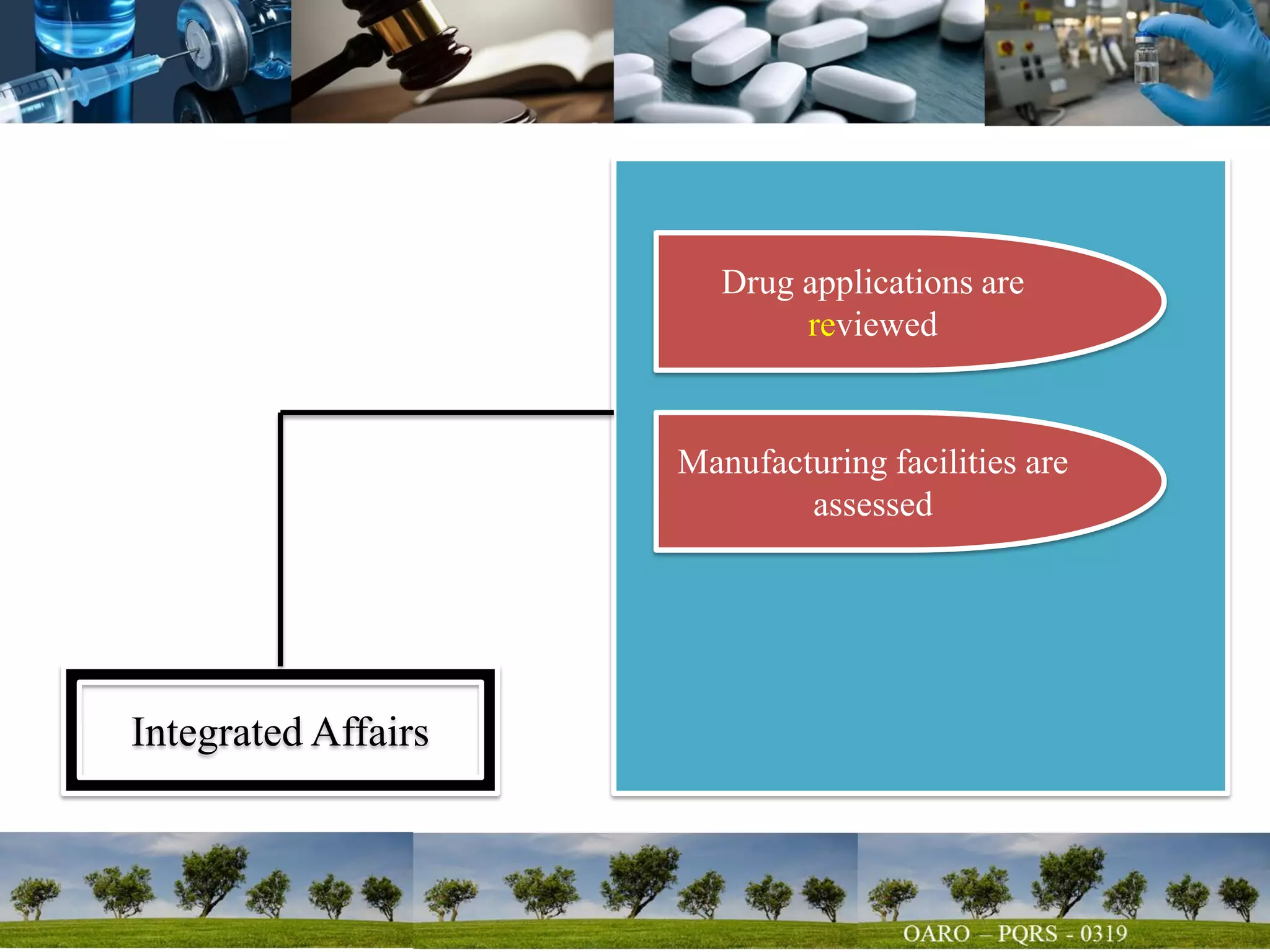
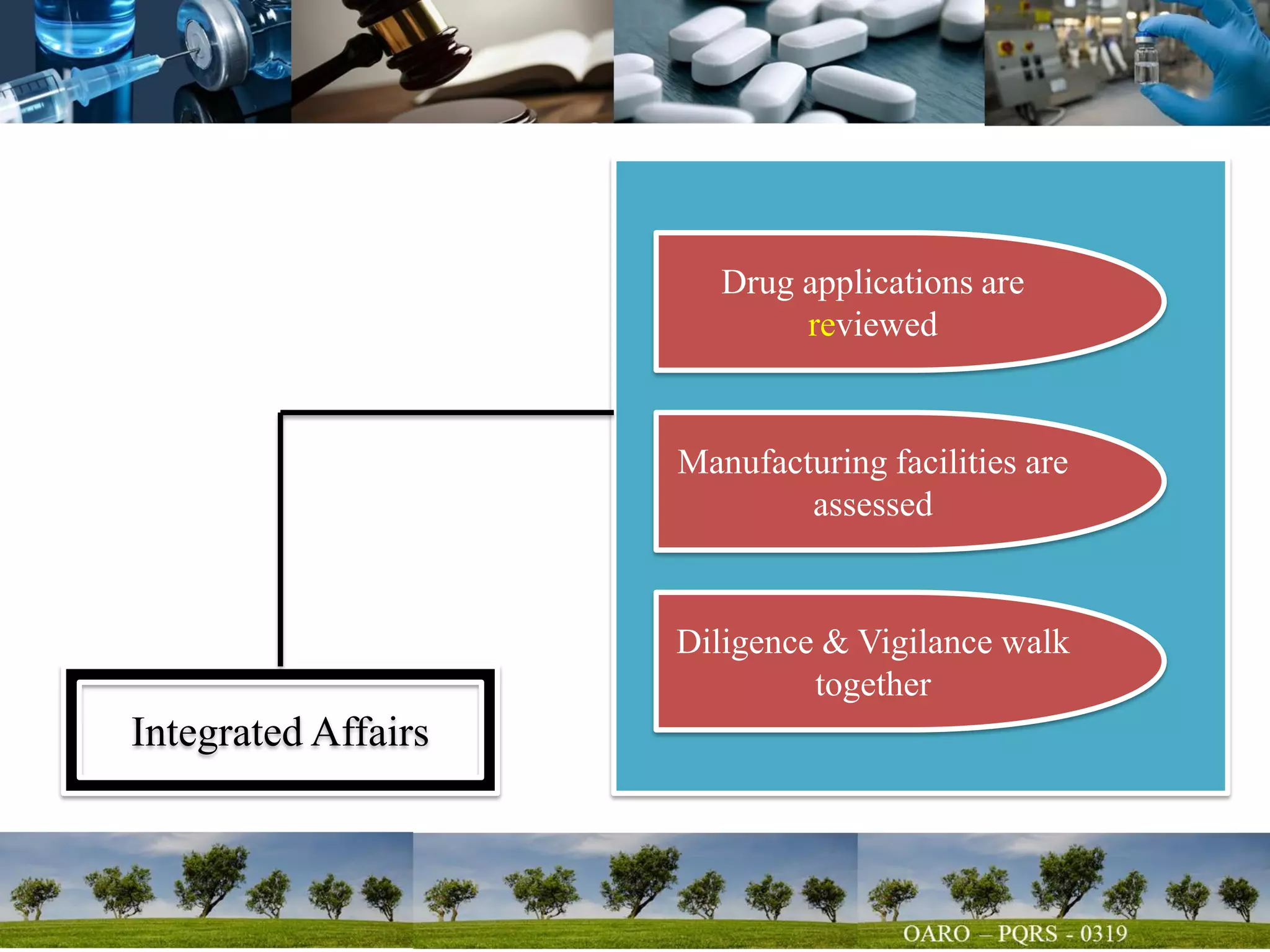
This document summarizes key points from a talk given to pharmacy students on the science behind drug quality. It discusses how drugs are manufactured, tested, and produce their intended effects. It also covers integrated affairs like drug application reviews and facility assessments. Global affairs like international harmonization are discussed. Innovation topics include continuous manufacturing and quality by design. The document emphasizes that understanding manufacturing processes, analytical sciences, and other disciplines is crucial for maintaining consistent drug quality. It stresses the importance of safety, efficacy and quality throughout the drug development and manufacturing process.