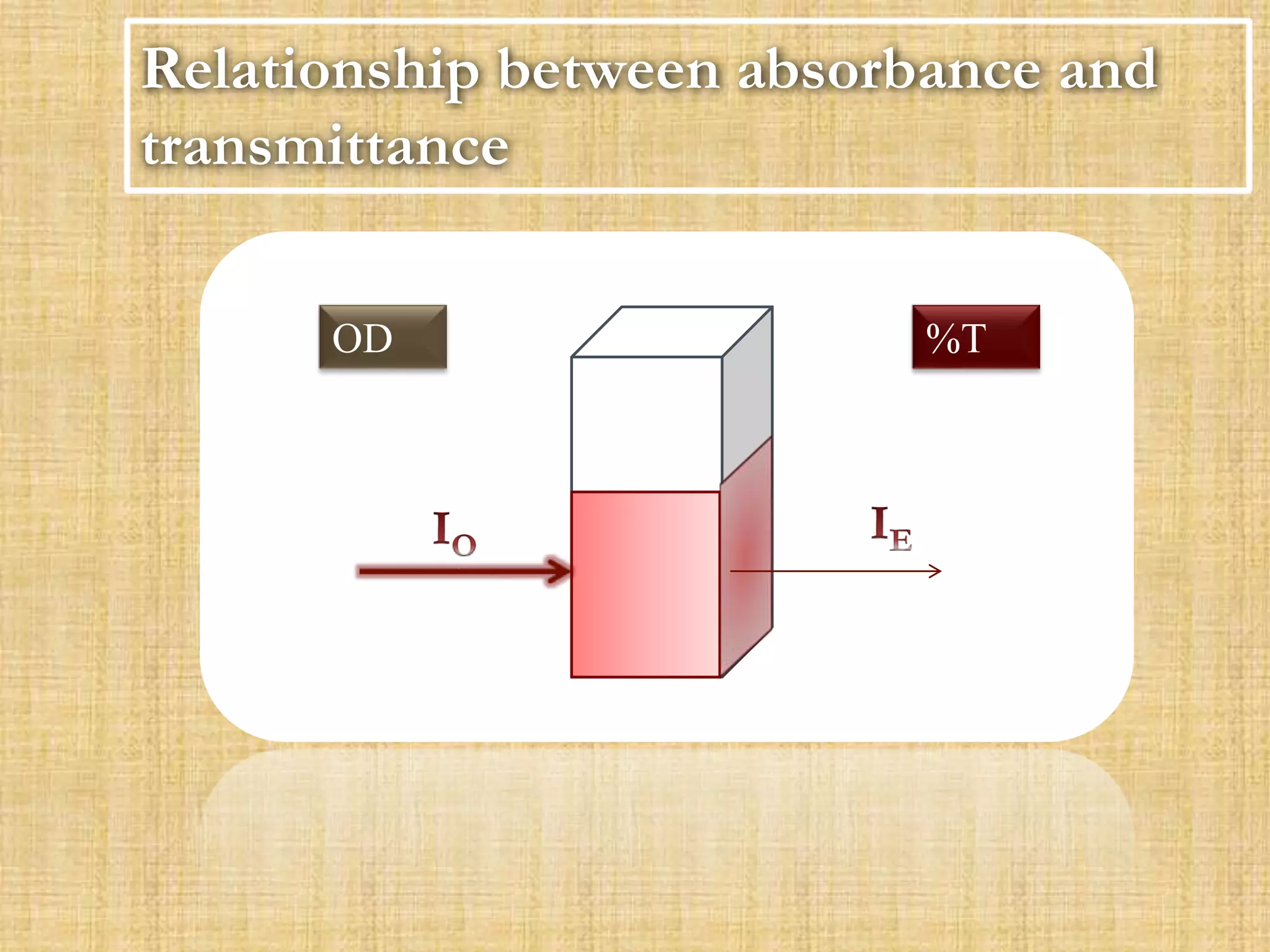
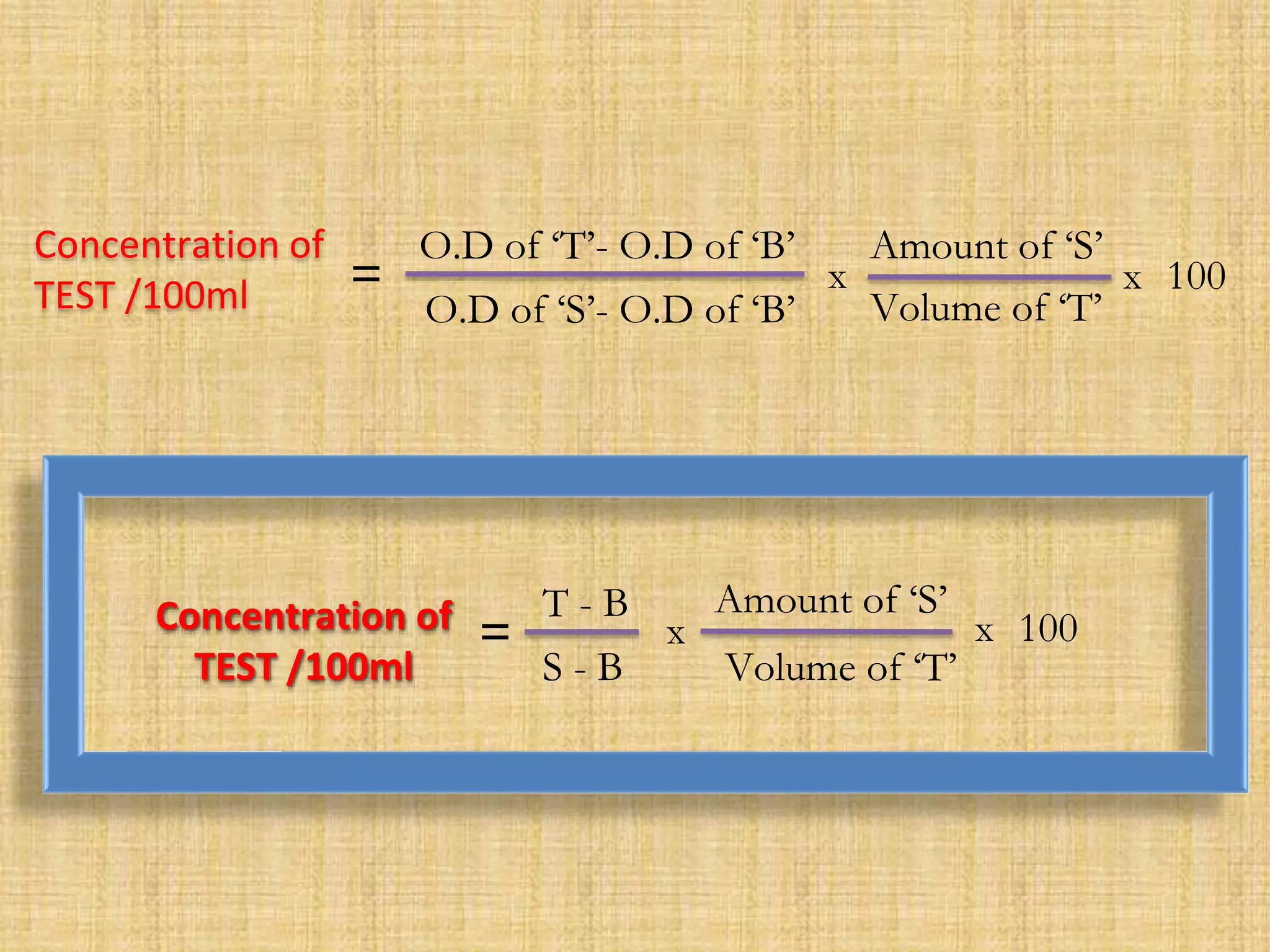
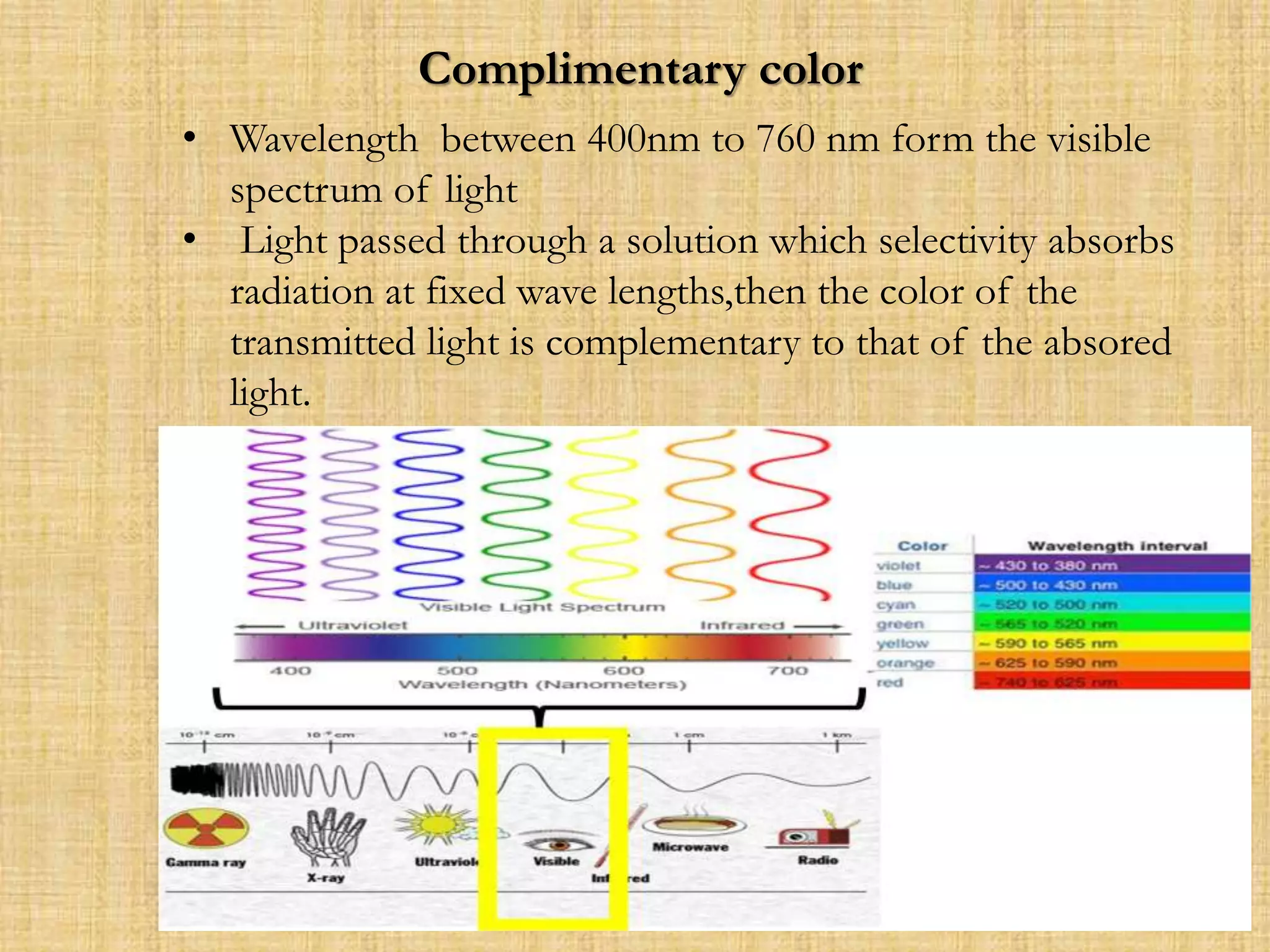
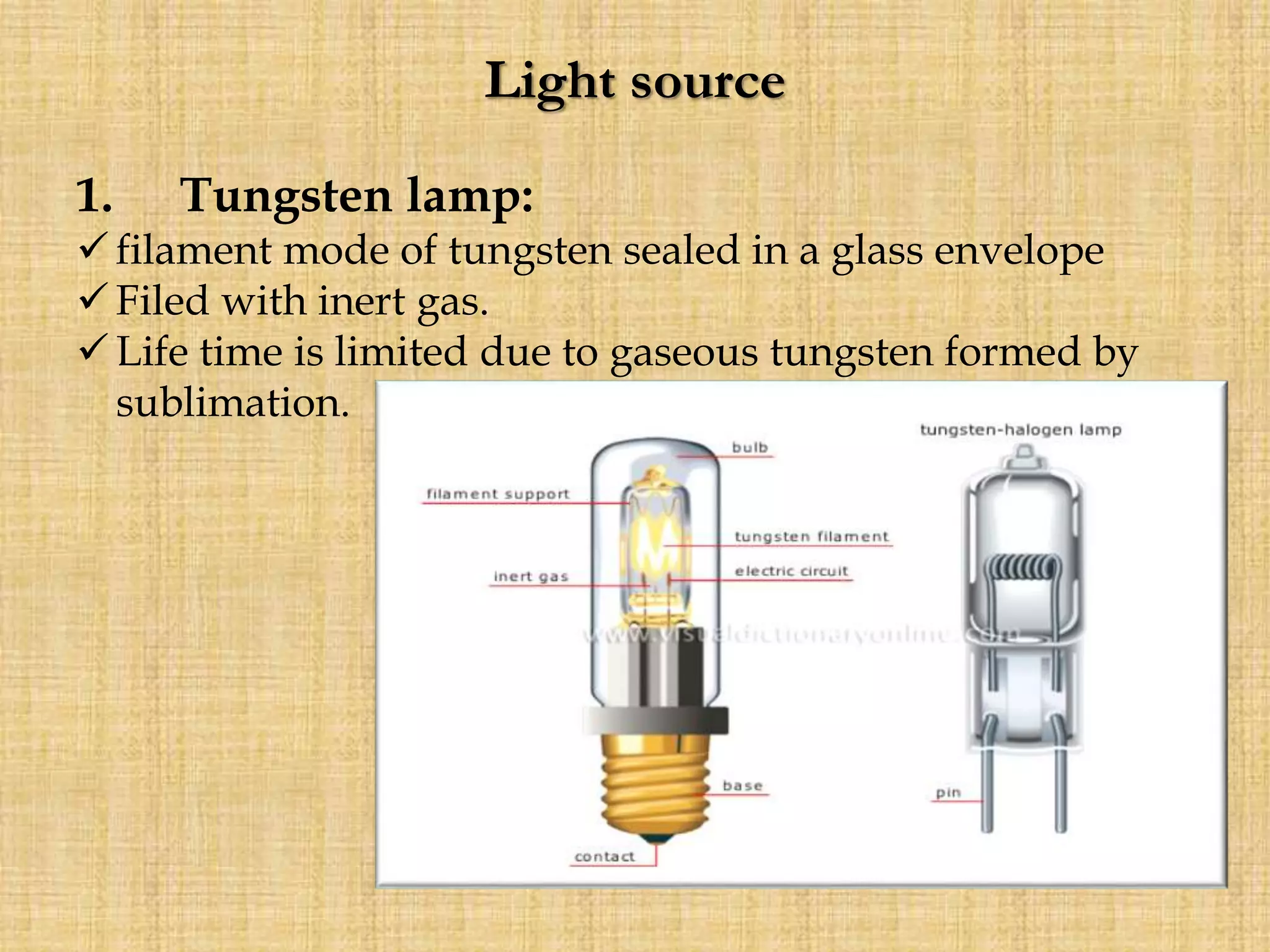
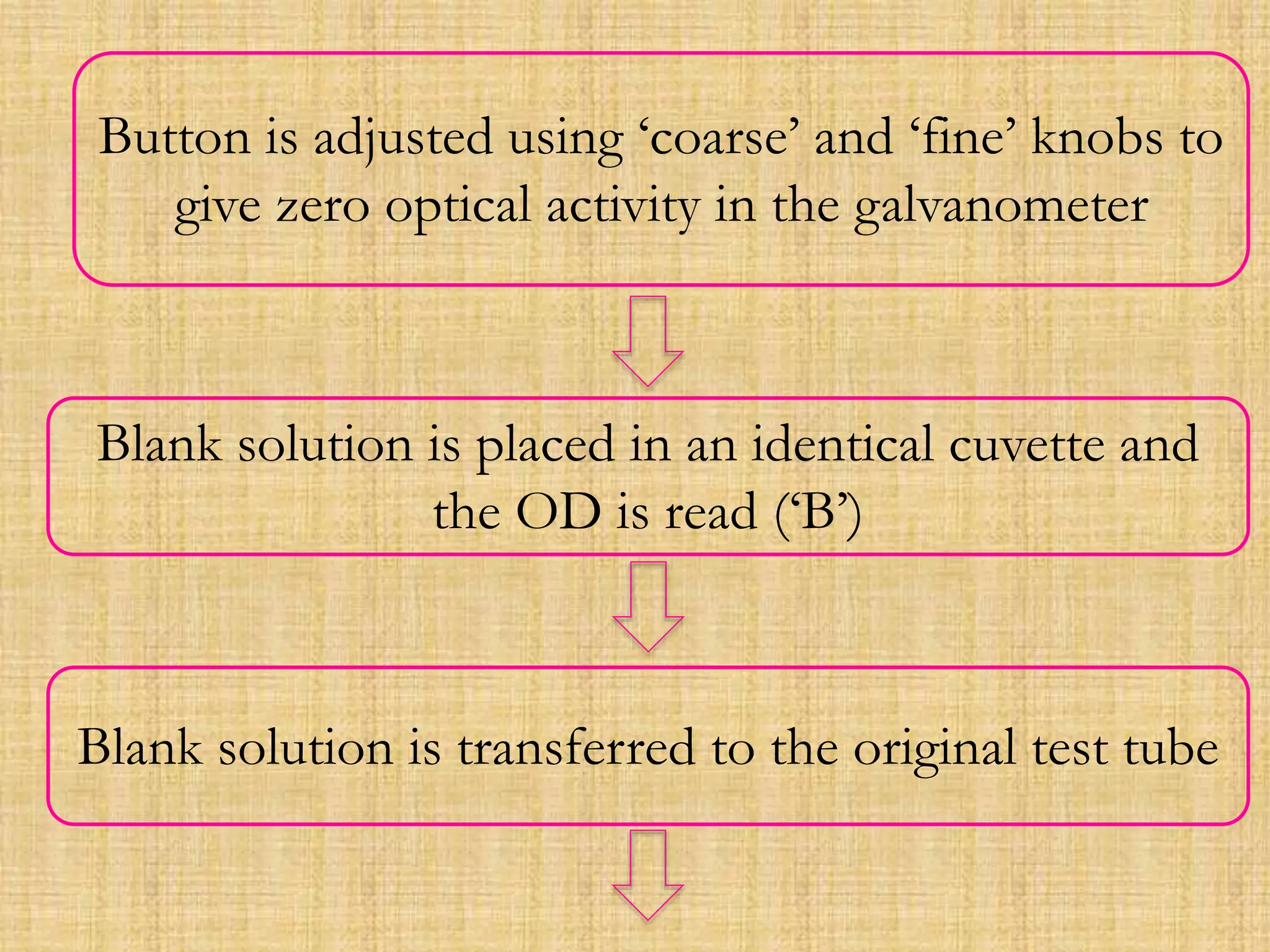
Photometry techniques like colorimetry, spectrophotometry, and turbidometry measure the intensity of light absorbed or transmitted by a solution. Colorimeters contain a light source, monochromators/filters to select wavelengths, a sample holder (cuvette), photodetectors, and readout devices. The amount of light absorbed follows Beer's and Lambert's laws - absorption increases exponentially with concentration and path length. A colorimeter is used to quantify compounds in biological samples like blood and urine by measuring absorbance and relating it to a standard curve using the Beer-Lambert law. Colorimeters provide a simple and inexpensive way to perform quantitative analysis of colored compounds.








































![Applications Of Colorimeter
• Estimation of biochemical compounds in blood, plasma,
serum, CSF, urine, etc.:
– Glucose
– Urea
– Creatinine
– Uric Acid
– Bilirubin
– Lipids
– Total Proteins
– Enzymes [e.g. ALT, AST, ALP]
– Minerals [Calcium, Phosphorus etc.] etc….](https://image.slidesharecdn.com/colorimetryclass2-161110120518/75/Colorimetry-class-46-2048.jpg)











