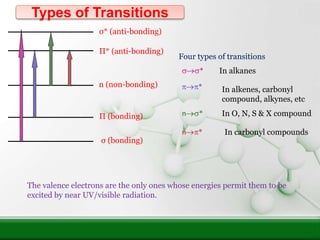
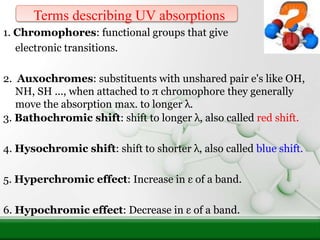
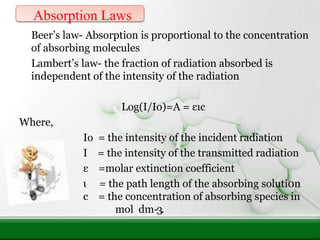
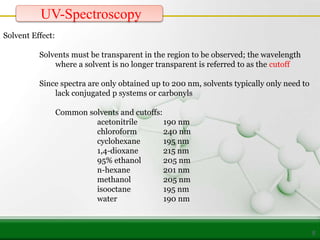
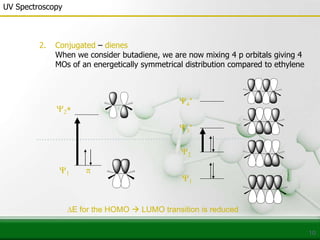
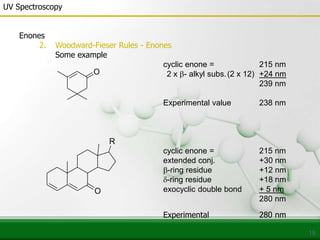
This document provides an overview of UV-visible spectroscopy and how it can be used to analyze conjugated systems. It discusses the different types of electronic transitions that can occur, and how solvents, conjugation, and substituents affect absorption properties. Woodward-Fieser rules are presented for predicting absorption maxima of conjugated dienes and α,β-unsaturated carbonyl compounds based on their structural features. Examples are given to demonstrate how to apply the rules to calculate expected absorption wavelengths.