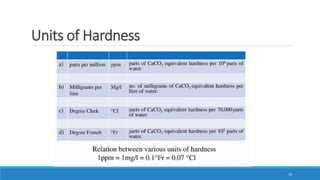
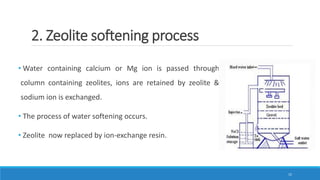
The document discusses the significance of water in the pharmaceutical industry, covering its physical and chemical properties, types of hardness, and methods for softening hard water. It explains different purification processes, including demineralization and sterilization for water used in injections, and details on the solubility of pharmaceuticals affected by various factors. It highlights the importance of selecting suitable water types based on requirements for usage in parenteral preparations.