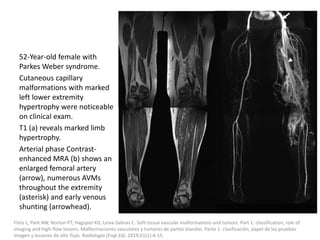
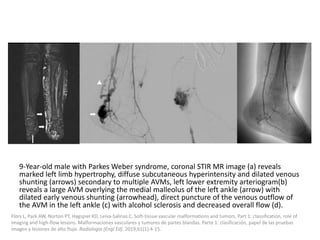
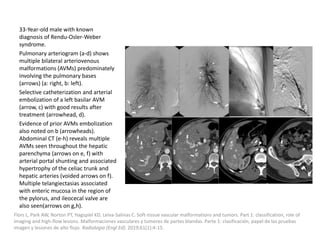
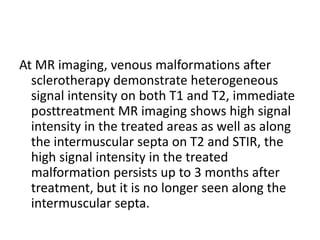
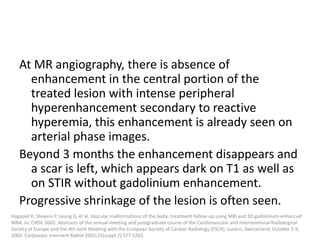
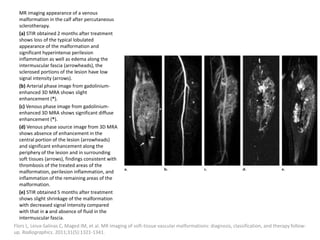
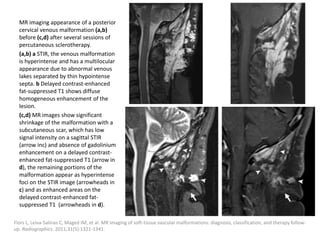
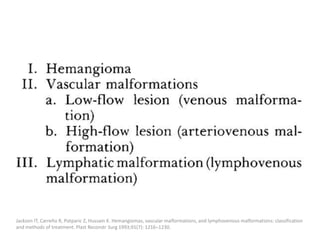
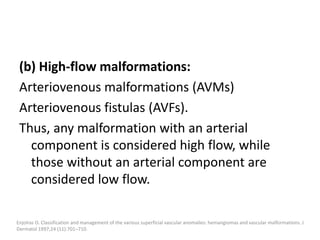
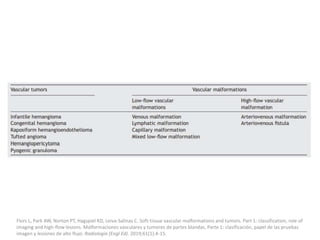
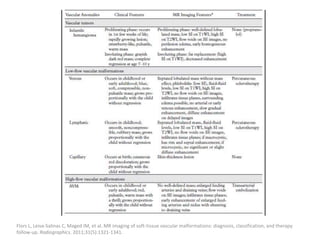
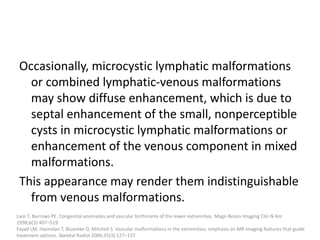
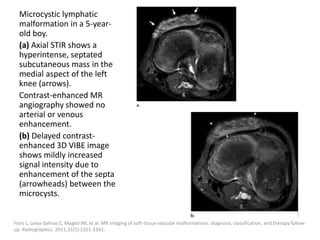
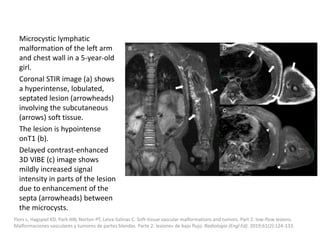
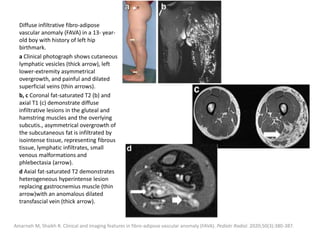
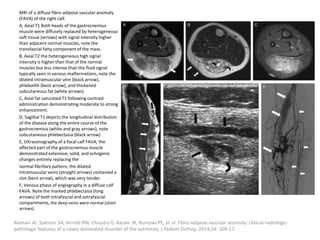
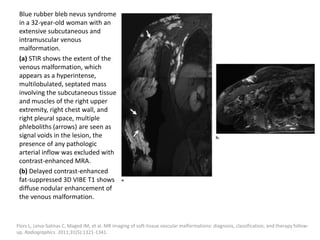
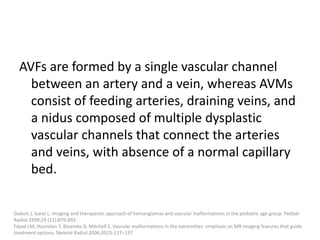
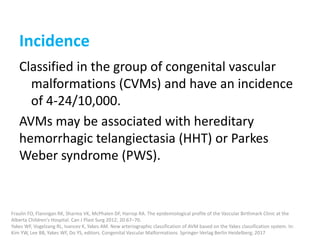
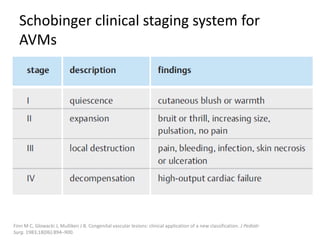
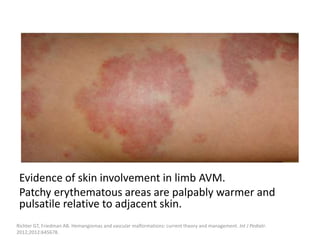
1) The document discusses vascular anomalies, specifically infantile hemangiomas.
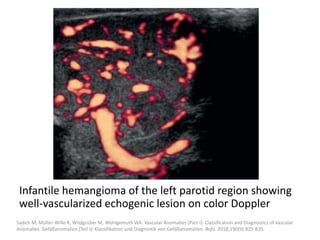
2) Infantile hemangiomas are the most common vascular tumor of infancy, affecting around 2-3% of children, most commonly on the face and neck.
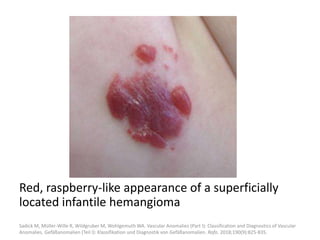
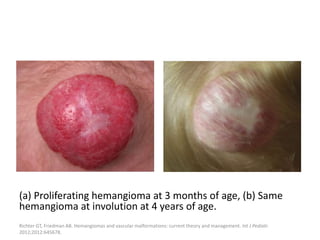
3) They typically appear as red, raised lesions within the first few months of life and then slowly involute over 5-10 years, leaving residual discoloration or texture changes to the skin.



















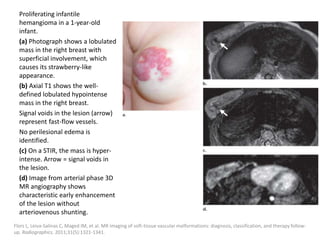
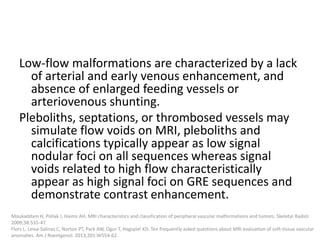
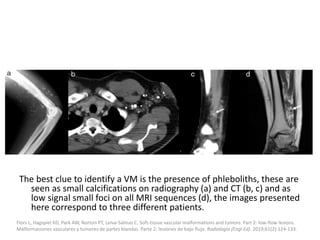
![b) MRI: Differ according to the biologic phase.
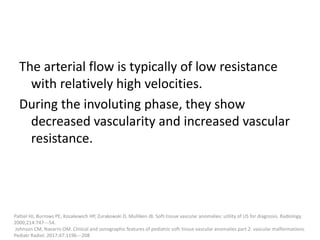
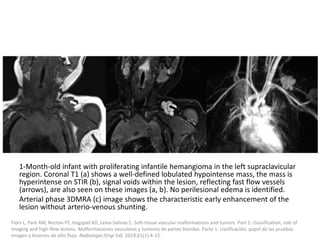
Proliferating phase:
they present as well-defined masses, hypointense
on T1 and hyperintense on T2, often with
presence of internal flow voids on spin-echo (SE)
imaging reflecting high-flow vessels.
High-flow vessels appear hyperintense on GRE
permitting the distinction with phlebolitis or
other calcifications, which are hypointense on all
imaging sequences.
. Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations:
diagnosis, classification, and therapy follow-up. Radiographics. 2011;31:321---40 [discussion 1340-1].](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-35-320.jpg)




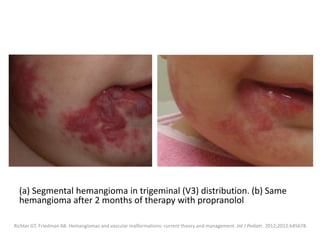
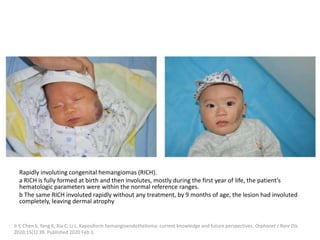
![Medical treatment is usually attempted first,
with propranolol used as a first-line therapy
with excellent results.
it reduces the expression of VEGF and other
proangiogenic factors while also inducing
apoptosis of vascular endothelial cells;
excellent results have been reported.
Léauté-Labrèze C, Taïeb A. Efficacy of betablockers in infantile capillary haemangiomas: the physiopathological significance and
therapeutic consequences [in French]. Ann Dermatol Venereol 2008;135(12):860–862.
Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 2010;40(6): 895–905.
Mulligan PR, Prajapati HJ, Martin LG, Patel TH. Vascular anomalies: classification, imaging characteristics and implications for
interventional radiology treatment approaches. Br J Radiol. 2014;87:20130392.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-43-320.jpg)





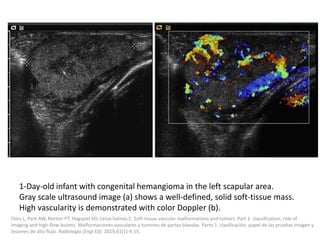
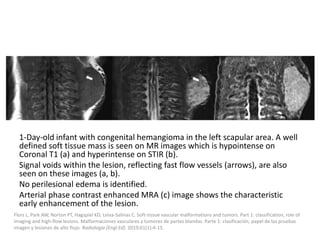






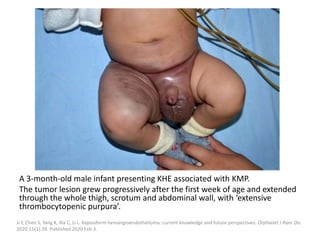
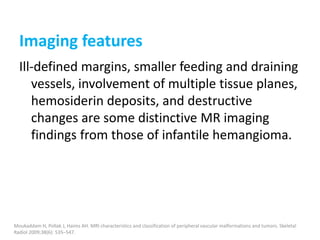
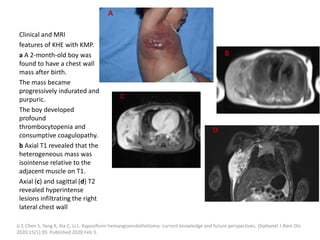





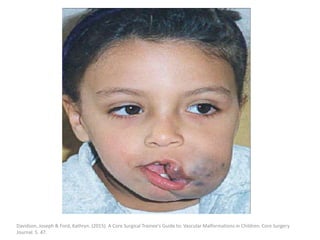
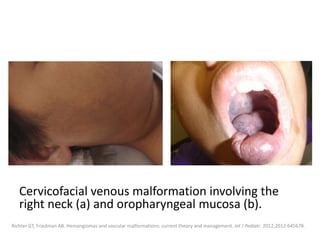


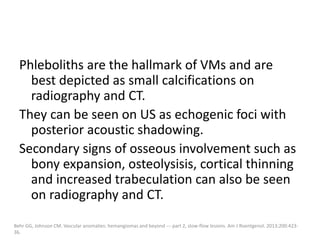
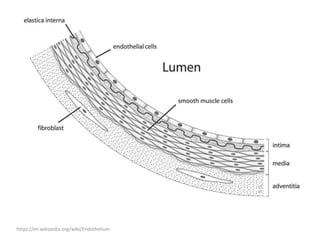
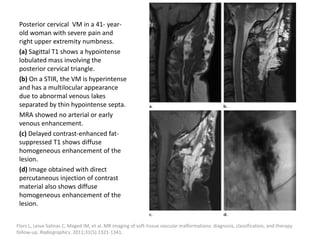
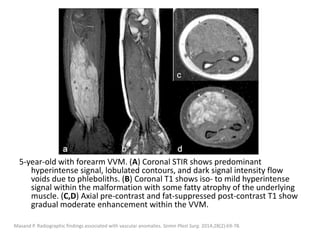
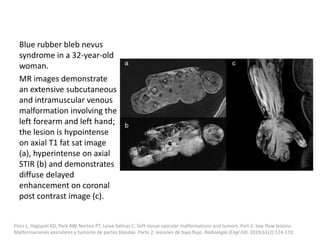
![b) MRI:
Present as lobulated, non-mass like lesions with
low to intermediate signal intensity on T1 and
hyperintensity on T2 and STIR.
Occasionally, hemorrhage or high protein
content may cause internal fluid-fluid levels.
In cases of thrombosis or hemorrhage,
heterogeneous signal intensity can be
observed on T1.
Ernemann U, Kramer U, Miller S, et al. Current concepts in the classification, diagnosis and treatment of vascular anomalies. Eur J
Radiol 2010;75 (1):2–11.
Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations:
diagnosis, classification, and therapy follow-up. Radiographics. 2011;31:1321-40 [discussion 1340-1].](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-79-320.jpg)
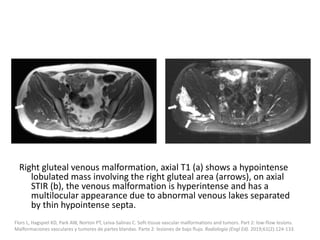
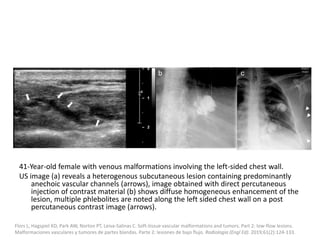
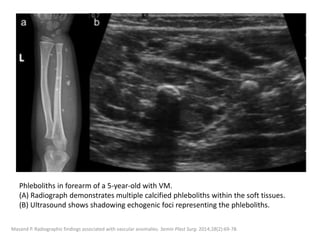
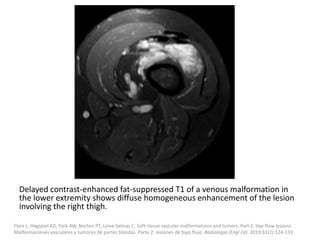
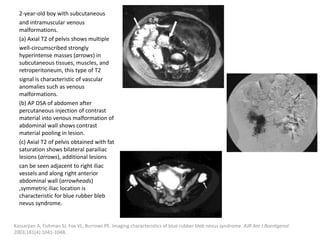
![VMs may infiltrate multiple tissue planes and fat
suppressed T2 and STIR provide excellent
delineation of the extension of the lesions.
Contrast administration is helpful and often
shows slow, gradual, delayed heterogeneous
contrast filling with characteristic diffuse
enhancement of the slow flowing venous
channels on delayed post-contrast T1.
Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol. 2010;40:895-905.
Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations:
diagnosis, classification, and therapy follow-up. Radiographics. 2011;31:1321---40 [discussion 1340-1].](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-81-320.jpg)
![Treatment
Conservative management
Sclerotherapy
Endovenous ablation techniques
Post-procedural care
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-88-320.jpg)








![Polidocanol foam
Preparation of
Aethoxysklerol foam.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-97-320.jpg)



![Venous malformation
(a) STIR sequence showed
a hyperintense venous
malformation with central
phleboliths (arrow)
(b) Percutaneous
puncture of the venous
malformation.
(c) Injection of contrast
media into the venous
malformation
(d) Injection of Polidocanol
foam.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-102-320.jpg)
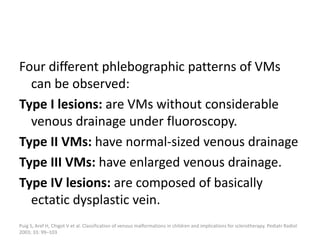
![Classification of venous
malformations
Type I lesions are
isolated
malformations
without
phlebographically
visible venous
drainage.
Type II lesions
demonstrate normal-
sized venous drainage.
Type III lesions
demonstrate enlarged
venous drainage.
Type IV lesions are
composed of
essentially ectatic
dysplastic veins.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-104-320.jpg)

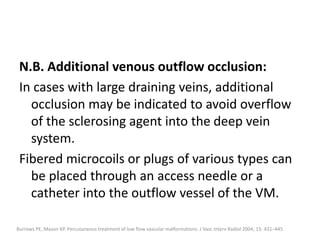
![Additionally, local compression of visible
draining veins may be considered.
In some cases it is necessary to puncture the VM
more than once to treat the lesion completely.
However, injection has to be stopped if there is
increased resistance, extravasation of the
sclerosant, or skin blanching.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-106-320.jpg)

![Post-procedural care
Patients should wear their compression garments
to help involution of the lesion.
Limb elevation, ice packs, and pain medication (an
NSAID is normally sufficient) may be indicated.
To prevent deep vein thrombosis (DVT),
prophylactic anticoagulation with LMWH is
recommended.
Ultrasound should be performed to exclude DVT
one day after therapy of limb VMs.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-109-320.jpg)




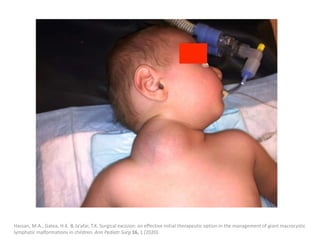
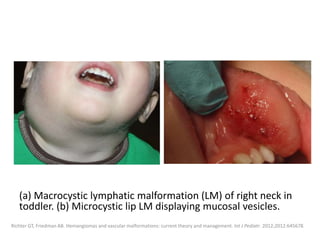
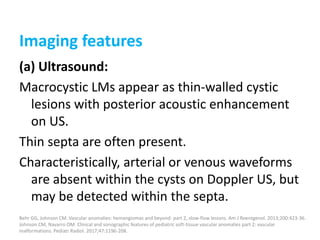

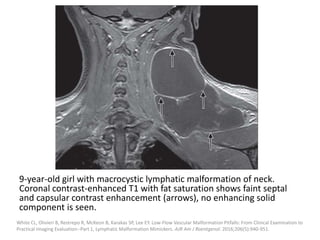
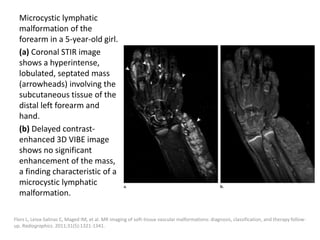
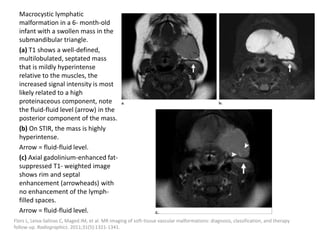
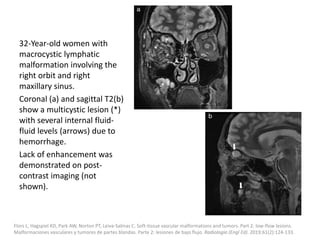








![Applications techniques
Cysts are cannulated with a needle under real-time
ultrasound guidance.
Alternatively, a pigtail catheter (3 to 5 French) can
be inserted in larger cysts, as the multiple side
holes facilitate aspiration of the lymphatic fluid
before injection of the sclerosing agent.
Contrast media can be injected to visualize the
whole lesion under fluoroscopy.
After aspiration of the entire cyst content, the LM
can then be treated with the sclerosant.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-142-320.jpg)



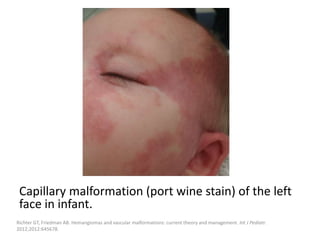
![Clinical presentation
Unlike VMs, CMs are usually present as a
macular pink to dark red patch with irregular
borders without bruit or local warmth.
Like LMs, they are usually localized in the head
and neck region.
Symptoms may be the result of deeper
associated malformations.
Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast
Reconstr Surg. 1982;69:412-22.
Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg.
1999;104:1-11 [discussion 12-5].
Fayad LM, Hazirolan T, Bluemke D, Mitchell S. Vascular malformations in the extremities: emphasis on MR imaging features that guide treatment options.
Skeletal Radiol. 2006;35: 127-37.
Ernemann U, Kramer U, Miller S, Bisdas S, Rebmann H, Breuninger H, et al. Current concepts in the classification, diagnosis and treatment of vascular
anomalies. Eur J Radiol. 2010;75:2-11.
Behr GG, Johnson CM. Vascular anomalies: hemangiomas and beyond -part 2, slow-flow lesions. Am J Roentgenol. 2013;200:423-36.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-147-320.jpg)




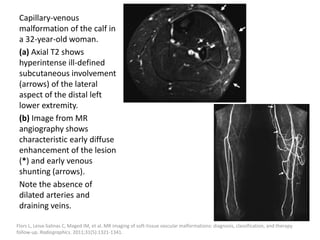
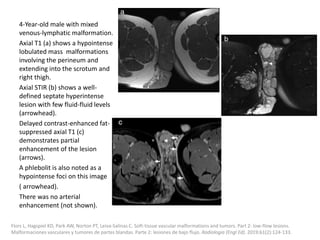




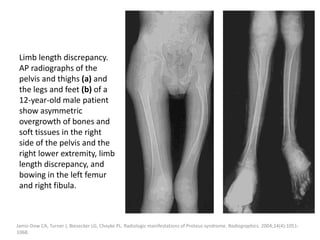
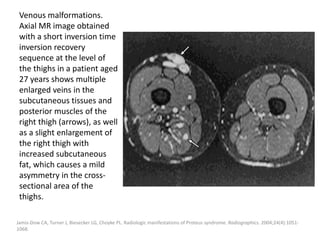

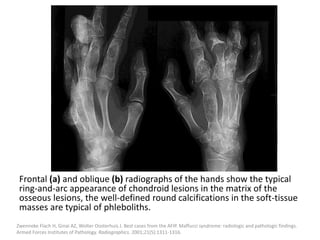
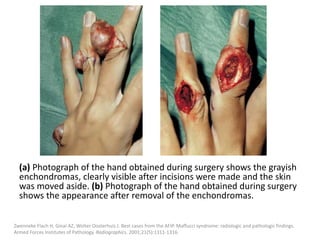






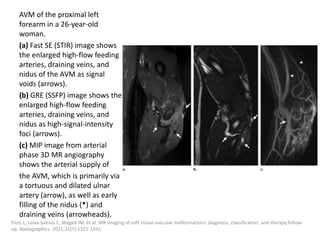
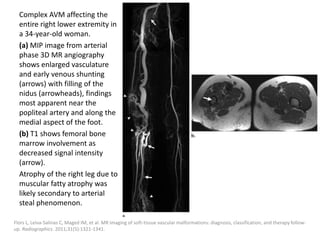
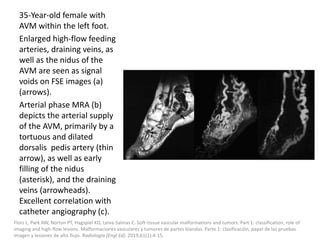
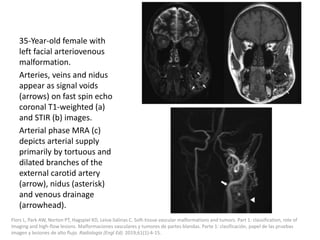




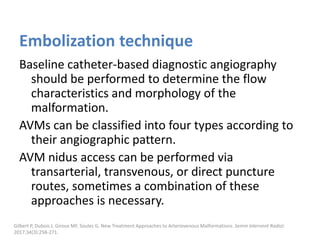
![The goal of endovascular embolotherapy is to
occlude the nidus or fistula completely.
Commonly used agents are:
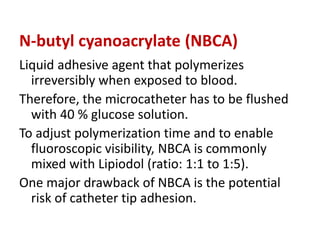
Ethanol
N-butyl cyanoacrylate (NBCA)
Ethylene-vinyl-alcohol-copolymer (EVOH)
Coils and plugs
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-208-320.jpg)

![Because of its low viscosity, ethanol passes the
nidus very quickly into the lung circulation.
Therefore, the pulmonary arterial pressure (PAP)
should be monitored continuously during ethanol
application.
PAP above 25mmHg systolic can be found 10 to 15
minutes after application.
To avoid side effects, it is preferred to administer
less than 0.5 ml per kg bodyweight in small
aliquots.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-210-320.jpg)
![As the liquid agent strictly follows the blood
flow, it is rarely possible to occlude the
complete nidus in large peripheral AVMs.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-212-320.jpg)


![Ethylene-vinyl-alcohol-copolymer (EVOH), EVOH is a non-adhesive liquid
embolic agent mixed with dimethyl sulfoxide (DMSO) and radiopaque
tantalum powder.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-215-320.jpg)
![EVOH (*) can be administered in a controlled manner under fluoroscopy, the
distribution of EVOH can be followed easily using the “road map” technique
(arrow).
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-216-320.jpg)
![Plug and push
technique.
Using the reflux of
EVOH as a plug around
the detachable tip of
the microcatheter, an
active forward flow of
EVOH into the whole
nidus is possible
regardless of the flow
direction.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-217-320.jpg)
![Plugs and coils
Coils or vascular plugs are needed in some cases to
optimize hemodynamics for further treatment,
but those only occlude the feeding vessels and
never reach the actual nidus, therefore, their use
is considered as adjuvant, and mere coiling of
AMVs is obsolete nowadays.
Plugs and coils can be used in simple structured
AVMs (type 1), for example in pulmonary fast-flow
malformations, they also have a role as an embolic
agent for outflow occlusion (type II lesions).
Wohlgemuth WA, Muller-Wille R, Teusch VI et al. The retrograde transvenous push-through method: a novel treatment of peripheral
arteriovenous malformations with dominant venous outflow. Cardiovasc Intervent Radiol 2015; 38: 623–63
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-218-320.jpg)
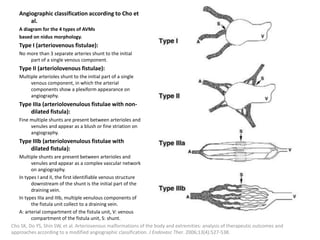
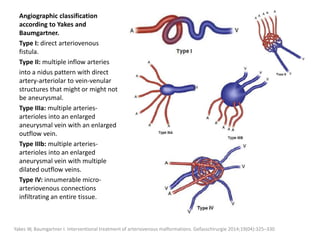

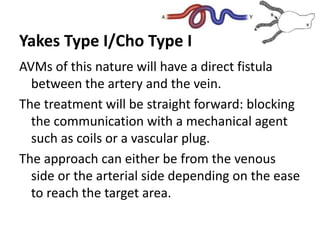

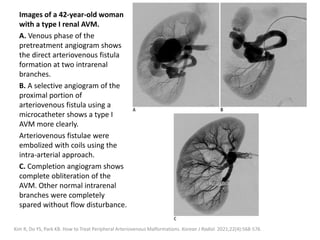
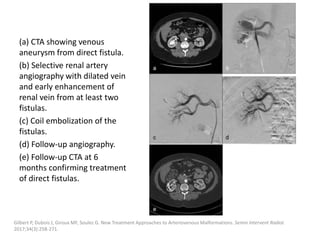
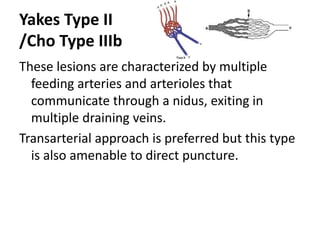



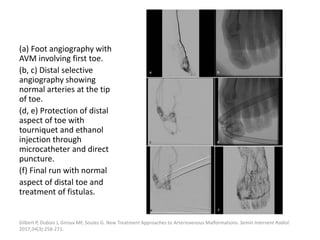


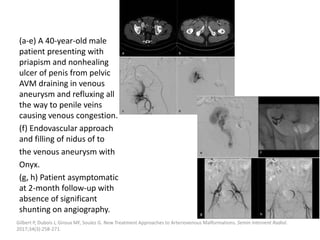
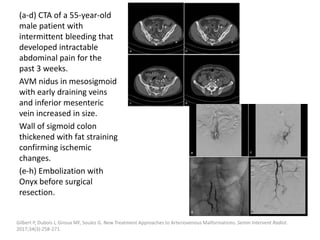


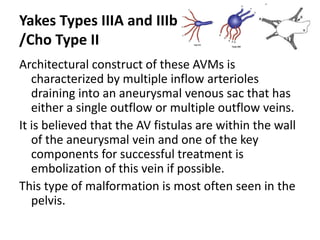


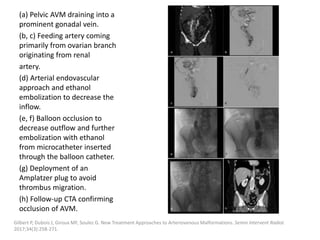
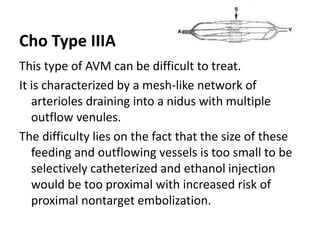

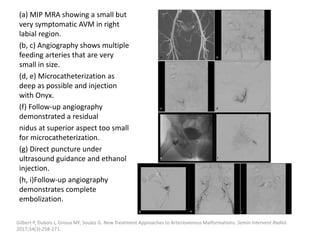
![Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-246-320.jpg)

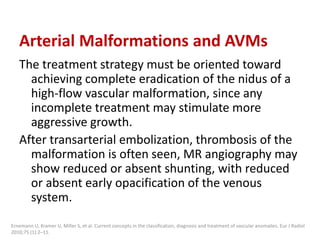
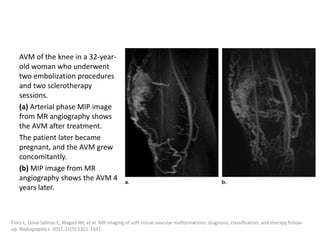
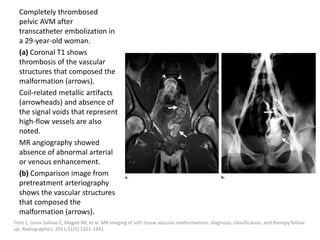
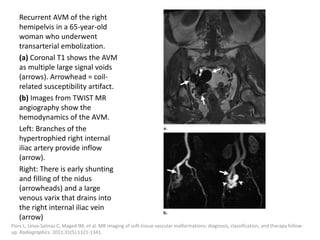
![After embolization, long-term clinical
surveillance with intermittent imaging should
be performed to rule out recurrence.
Incomplete nidus embolization may stimulate
aggressive growth.
Müller-Wille R, Wildgruber M, Sadick M, Wohlgemuth WA. Vascular Anomalies (Part II): Interventional Therapy of Peripheral Vascular
Malformations [published online ahead of print, 2018 Feb 7]. Gefäßanomalien (Teil II): Interventionelle Therapie von peripheren
Gefäßmalformationen [published online ahead of print, 2018 Feb 7]. Rofo. 2018;10.1055/s-0044-101266.](https://image.slidesharecdn.com/vascularanomalies-220929223146-3387dd4d/85/Vascular-anomalies-pptx-248-320.jpg)