Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature. It relates to the evaporation rate of a liquid and is affected by factors like temperature, composition of mixtures, and presence of solids or liquids. Vapor pressure increases non-linearly with temperature and the boiling point of a liquid is reached when its vapor pressure equals atmospheric pressure. It plays an important role in cloud formation through processes like condensation, supersaturation, and Raoult's law governing vapor pressures in mixtures.
![substance with a high vapor pressure at normal temperatures is often referred to as volatile. The
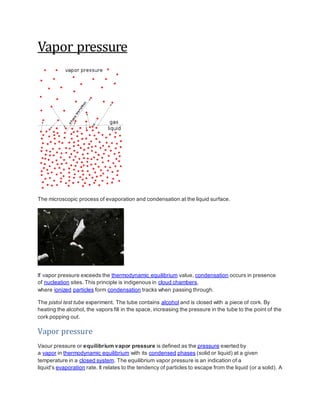
pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the
temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic
energy of the molecules increases, the number of molecules transitioning into a vapor also
increases, thereby increasing the vapor pressure.
The vapor pressure of any substance increases non-linearly with temperature according to
the Clausius–Clapeyron relation. The atmospheric pressure boiling point of a liquid (also known as
the normal boiling point) is the temperature at which the vapor pressure equals the ambient
atmospheric pressure. With any incremental increase in that temperature, the vapor pressure
becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapor bubbles inside
the bulk of the substance. Bubble formation deeper in the liquid requires a higher temperature due to
the higher fluid pressure, because fluid pressure increases above the atmospheric pressure as the
depth increases. More important at shallow depths is the higher temperature required to start bubble
formation. The surface tension of the bubble wall leads to an overpressure in the very small, initial
bubbles.
The vapor pressure that a single component in a mixture contributes to the total pressure in the
system is called partial pressure. For example, air at sea level, and saturated with water vapor at
20 °C, has partial pressures of about 2.3 kPa of water, 78 kPa of nitrogen, 21 kPa of oxygen and 0.9
kPa of argon, totaling 102.2 kPa, making the basis for standard atmospheric pressure.
Measurement and units[edit]
Vapor pressure is measured in the standard units of pressure. The International System of Units (SI)
recognizes pressure as a derived unit with the dimension of force per area and designates
the pascal (Pa) as its standard unit. One pascal is one newton per square meter (N·m−2 or
kg·m−1·s−2)
Relation to boiling point of liquids[edit]](https://image.slidesharecdn.com/janhavi-210512073512/85/vapour-pressure-2-320.jpg)
![A log-lin vapor pressure chart for various liquids
As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing
boiling points. This is illustrated in the vapor pressure chart (see right) that shows graphs of
the vapor pressures versus temperatures for a variety of liquids.[6] At the normal boiling point of a
liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1
atmosphere,[7] 760 Torr, 101.325 kPa, or 14.69595 psi.
For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the
liquids in the chart. It also has the lowest normal boiling point (−24.2 °C), which is where the vapor
pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one
atmosphere (atm) of absolute vapor pressure. Liquid mixtures: Raoult's law[edit]
Raoult's law
Raoult’slaw givesanapproximationtothe vaporpressure of mixturesof liquids.Itstatesthatthe
activity(pressure orfugacity) of a single-phase mixtureisequal tothe mole-fraction-weightedsumof
the components'vaporpressures:
where is the mixture's vapor pressure, is the mole fraction of component in the liquid phase and is
the mole fraction of component in the vapor phase respectively. is the vapor pressure of
component . Raoult's law is applicable only to non-electrolytes (uncharged species); it is most
appropriate for non-polar molecules with only weak intermolecular attractions Solids[edit]
Vapor pressure of liquid and solid benzene
Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in
equilibrium with its own vapor. In the case of an equilibrium solid, such as a crystal, this can be
defined as the pressure when the rate of sublimation of a solid matches the rate of deposition of its
vapor phase. For most solids this pressure is very low, but some notable exceptions](https://image.slidesharecdn.com/janhavi-210512073512/85/vapour-pressure-3-320.jpg)
![are naphthalene, dry ice (the vapor pressure of dry ice is 5.73 MPa (831 psi, 56.5 atm) at 20 °C,
which causes most sealed containers to rupture), and ice. All solid materials have a vapor pressure.
However, due to their often extremely low values, measurement can be rather difficult. Typical
techniques include the use of thermogravimetry and gas transpiration.
There are a number of methods for calculating the sublimation pressure (i.e., the vapor pressure) of
a solid. One method is to estimate the sublimation pressure from extrapolated liquid vapor pressures
(of the supercooled liquid), if the heat of fusion is known, by using this particular form of the
Clausius–Clapeyron relation:[9]
where:
is the sublimation pressure of the solid component at the temperature .
is the extrapolated vapor pressure of the liquid component at the temperature .
is the heat of fusion.
is the gas constant.
is the sublimation temperature.
is the melting point temperature.
This method assumes that the heat of fusion is temperature-independent, ignores additional
transition temperatures between different solid phases, and it gives a fair estimation for
temperatures not too far from the melting point. It also shows that the sublimation pressure is lower
than the extrapolated liquid vapor pressure (Δf usH > 0) and the difference grows with increased
distance from the melting.
Boiling point of water](https://image.slidesharecdn.com/janhavi-210512073512/85/vapour-pressure-4-320.jpg)

![Meaning in meteorology
In meteorology, the term vapor pressure is used to mean the partial pressure of water vapor in the
atmosphere, even if it is not in equilibrium,[15]
and the equilibrium vapor pressure is specified
otherwise. Meteorologists also use the term saturation vapor pressure to refer to the equilibrium
vapor pressure of water or brine above a flat surface, to distinguish it from equilibrium vapor
pressure, which takes into account the shape and size of water droplets and particulates in the
atmosphere.[16
Vapour pressure effects in cloud formation
Clouds droplets initially form by the condensation of water vapor onto condensation nuclei when
the supersaturation of air exceeds a critical value according to Köhler theory. Cloud condensation
nuclei are necessary for cloud droplets formation because of the Kelvin effect, which describes the
change in saturation vapor pressure due to a curved surface. At small radii, the amount of
supersaturation needed for condensation to occur is so large, that it does not happen
naturally. Raoult's law describes how the vapor pressure is dependent on the amount of solute in a
solution. At high concentrations, when the cloud droplets are small, the supersaturation required is
smaller than without the presence of a nucleus.](https://image.slidesharecdn.com/janhavi-210512073512/85/vapour-pressure-6-320.jpg)
![Supersaturation
The amount of water that can exist as vapor in a given volume increases with the temperature.
When the amount of water vapor is in equilibrium above a flat surface of water the level of vapor
pressure is called saturation and the relative humidity is 100%. At this equilibrium there are equal
numbers of molecules evaporating from the water as there are condensing back into the water. If th e
relative humidity becomes greater than 100%, it is called supersaturated. Supersaturation occurs in
the absence of condensation nuclei.
Since the saturation vapor pressure is proportional to temperature, cold air has a lower saturation
point than warm air. The difference between these values is the basis for the formation of clouds.
When saturated air cools, it can no longer contain the same amount of water vapor. If the conditions
are right, the excess water will condense out of the air until the lower saturation point is reached.
Another possibility is that the water stays in vapor form, even though it is beyond the saturation
point, resulting in supersaturation.
Supersaturation of more than 1–2% relative to water is rarely seen in the atmosphere, since cloud
condensation nuclei are usually present.[26] Much higher degrees of supersaturation are possible in
clean air, and are the basis of the cloud chamber.
REFERENCE
https://www.sciencedirect.com/topics/earth-and-planetary-sciences/vapour-pressure
https://www.chem.purdue.edu/gchelp/liquids/vpress.html
https://www.britannica.com/science/vapor-pressure
http://terpconnect.umd.edu/~wbreslyn/chemistry/pressure/index-pressure.html](https://image.slidesharecdn.com/janhavi-210512073512/85/vapour-pressure-7-320.jpg)