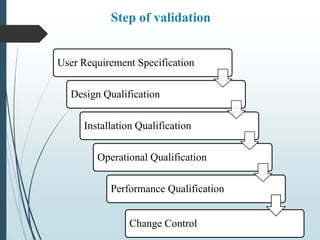
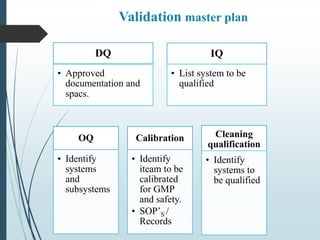
The validation master plan outlines the general approach and expectations for validation of a new pharmaceutical facility. It defines the scope of validation to include all production areas, storage, utilities, and staff facilities. The plan describes the user requirement specifications, design qualifications, installation qualifications, operational qualifications and performance qualifications that will be conducted. It identifies the processes and systems to be validated and the documentation, including protocols and reports, that will be generated. The plan also provides schedules, assigns responsibilities to personnel, and identifies training requirements to ensure the validation activities are properly executed and meet regulatory expectations.