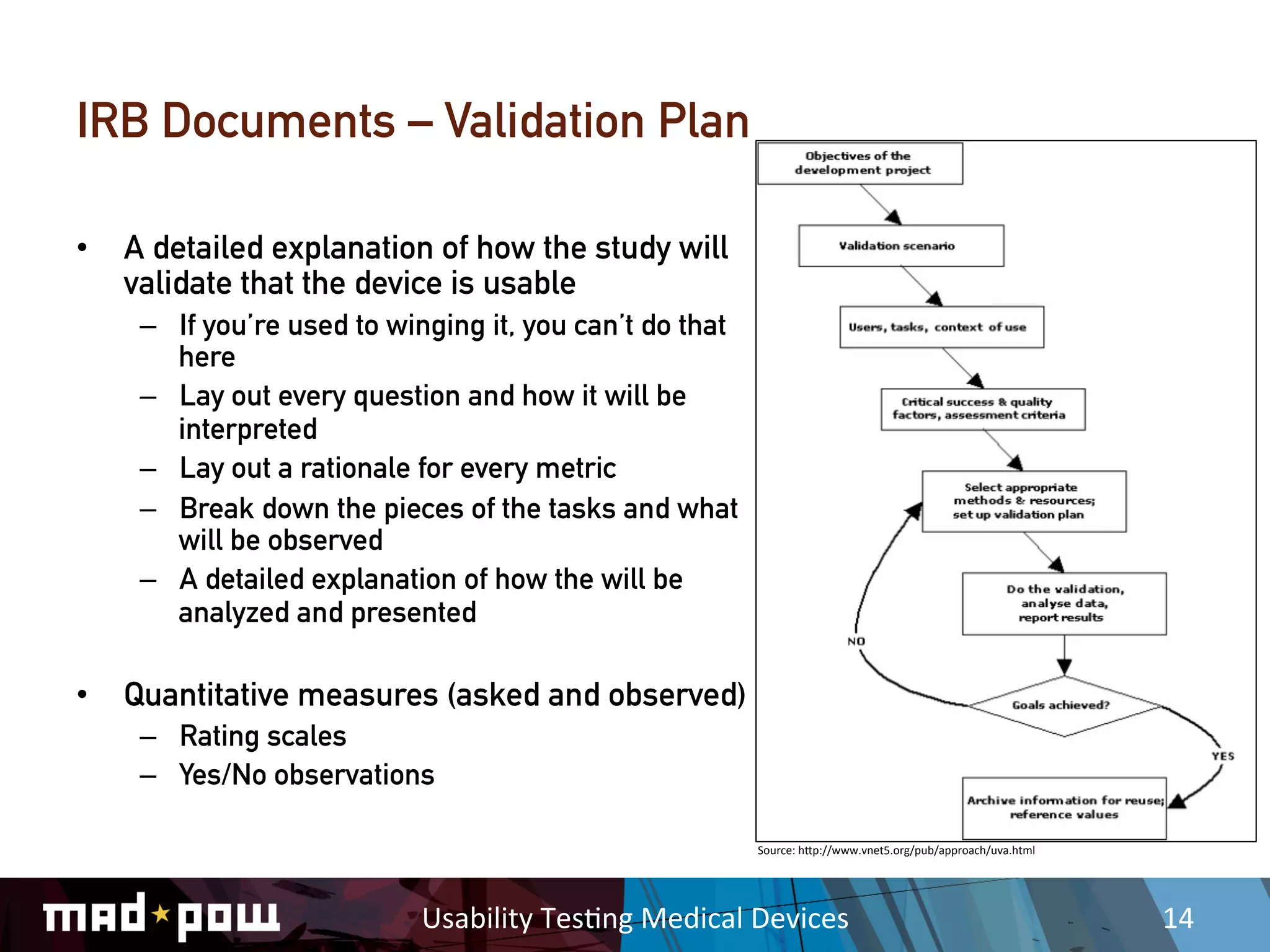
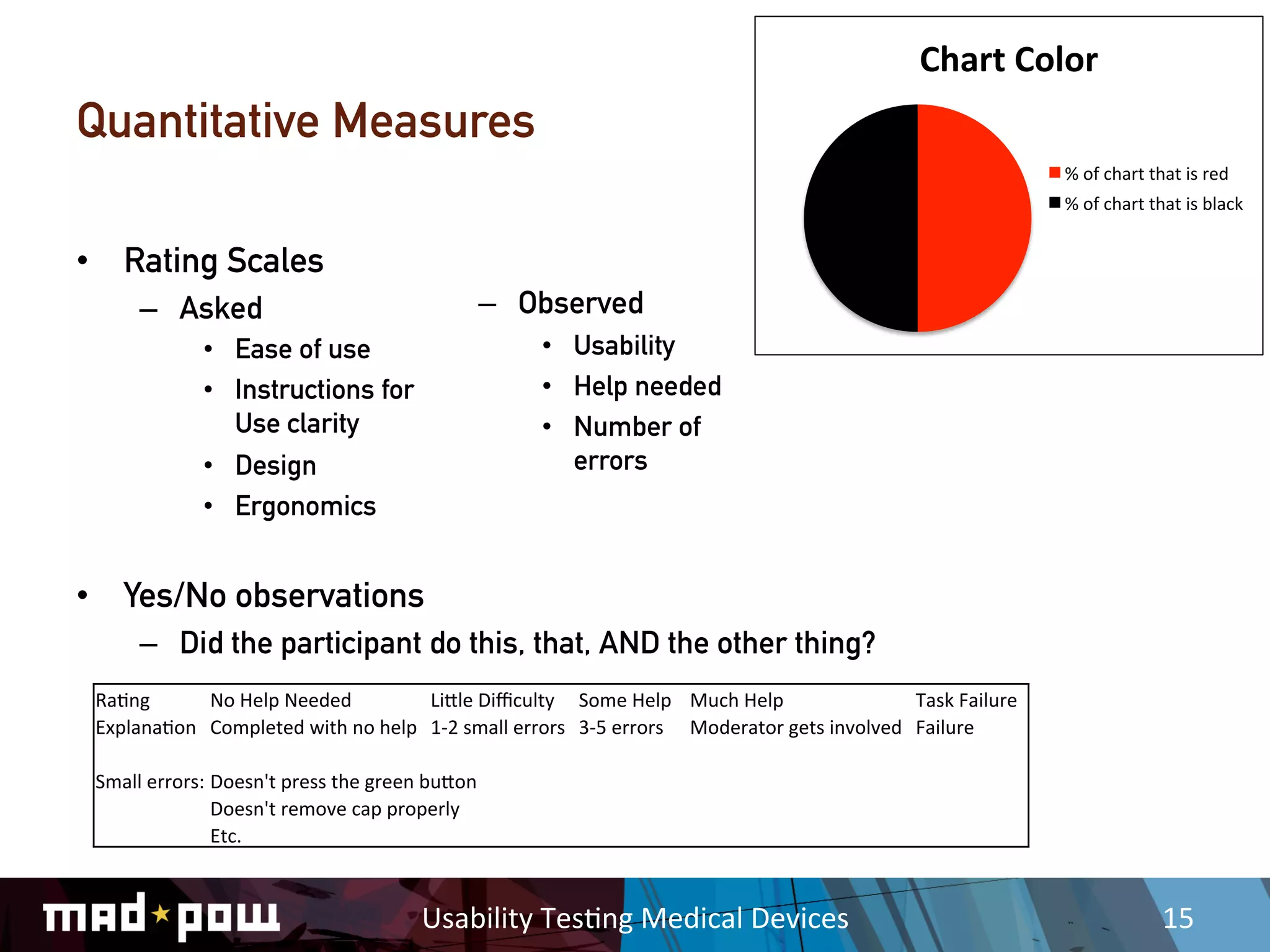
This document discusses key considerations for usability testing of medical devices. It notes that while usability activities are not clinical trials, they will still require extensive documentation and safety protocols. Researchers must understand relevant regulations and work closely with clients, medical experts, and Institutional Review Boards. Additional precautions are needed when testing with vulnerable participants or children. Moderator guides and data collection methods must be rigorously defined to meet regulatory requirements.