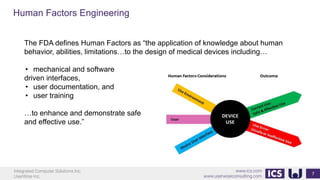
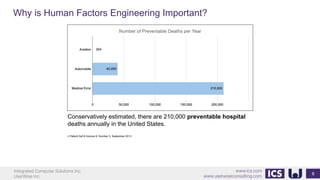
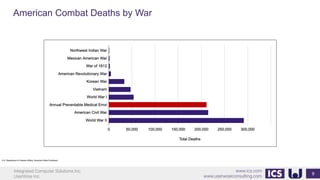
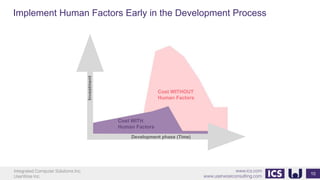
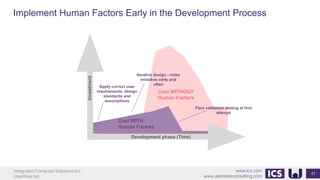
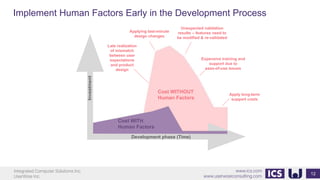
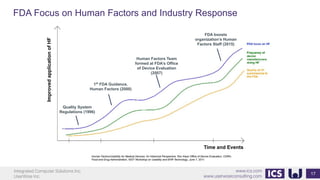
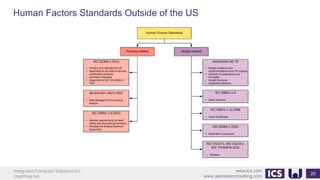
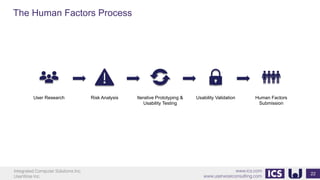
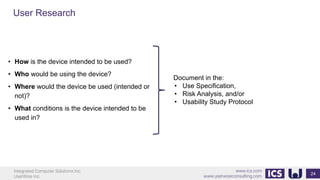
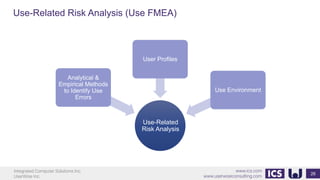
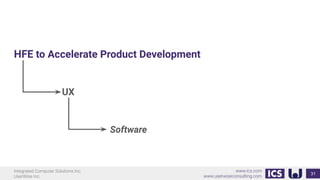
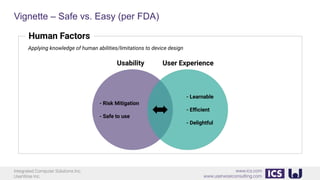
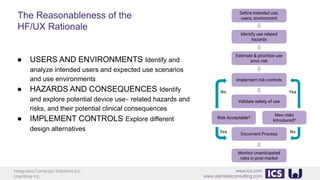
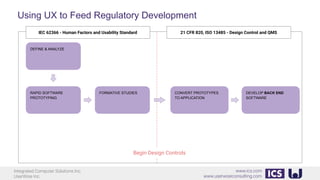
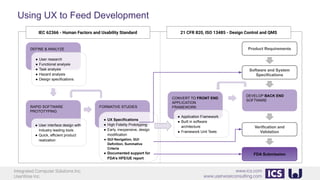
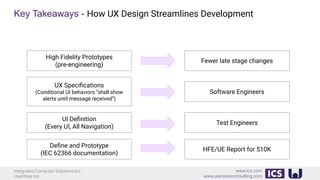
This document provides an introduction to human factors and user experience (UX) for medical devices. It discusses Integrated Computer Solutions Inc. and UserWise Inc., two companies that provide UX and human factors engineering services for medical products. The document outlines the human factors process, which includes user research, risk analysis, iterative prototyping and usability testing to develop usable and safe medical devices that are compliant with regulations. It emphasizes applying human factors early in the design process to reduce costs and facilitate regulatory approval.