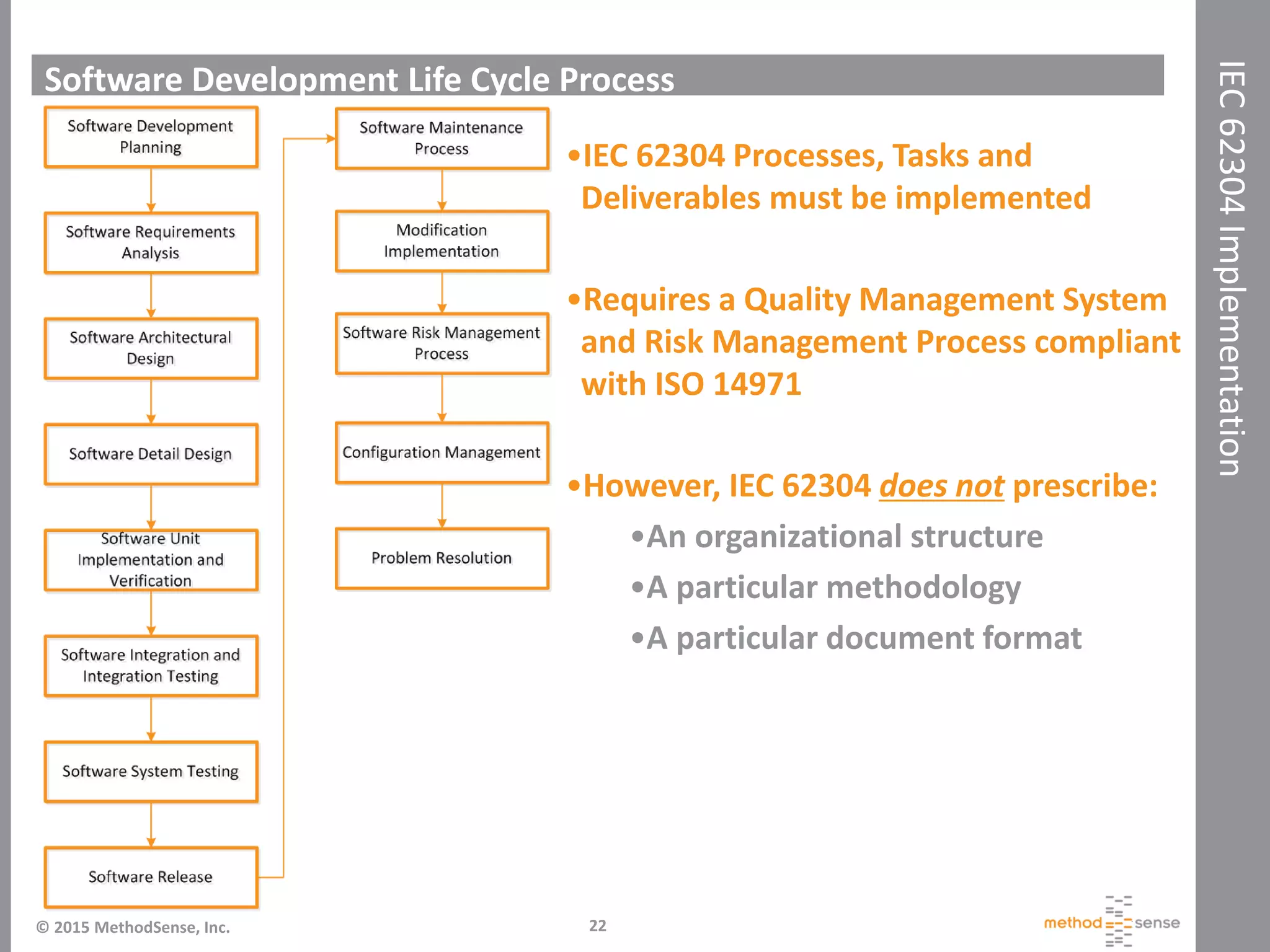
The document discusses IEC 62304, an international standard for software development lifecycle requirements specifically for medical device software. It emphasizes the importance of conformance to improve safety, manage risks, and fulfill regulatory obligations, especially in the EU and the U.S. The presentation also highlights the implementation challenges, software safety classifications, and best practices to ensure compliance with the standard.