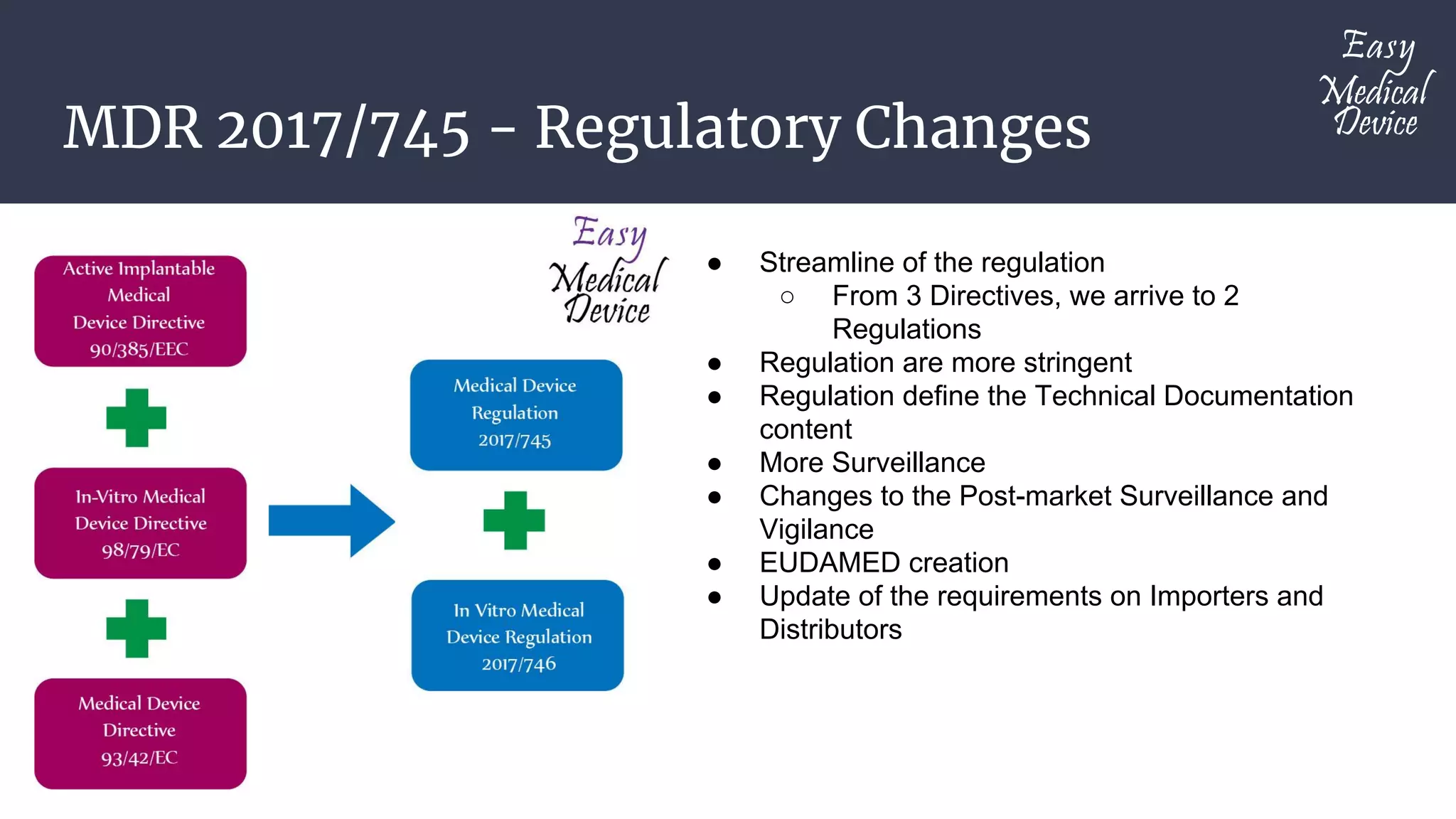
The document discusses the regulatory changes under MDR 2017/745, which aims to streamline regulations from three directives to two, introduces stricter requirements, and emphasizes improved post-market surveillance following the PIP breast implant scandal. It outlines the medical device classification system in Annex VIII, detailing four categories of devices and their corresponding risk classes, specifically highlighting Class I subclasses. Additionally, it notes that Class I products can be self-declared, while subclasses require notified body involvement for certain specifications.