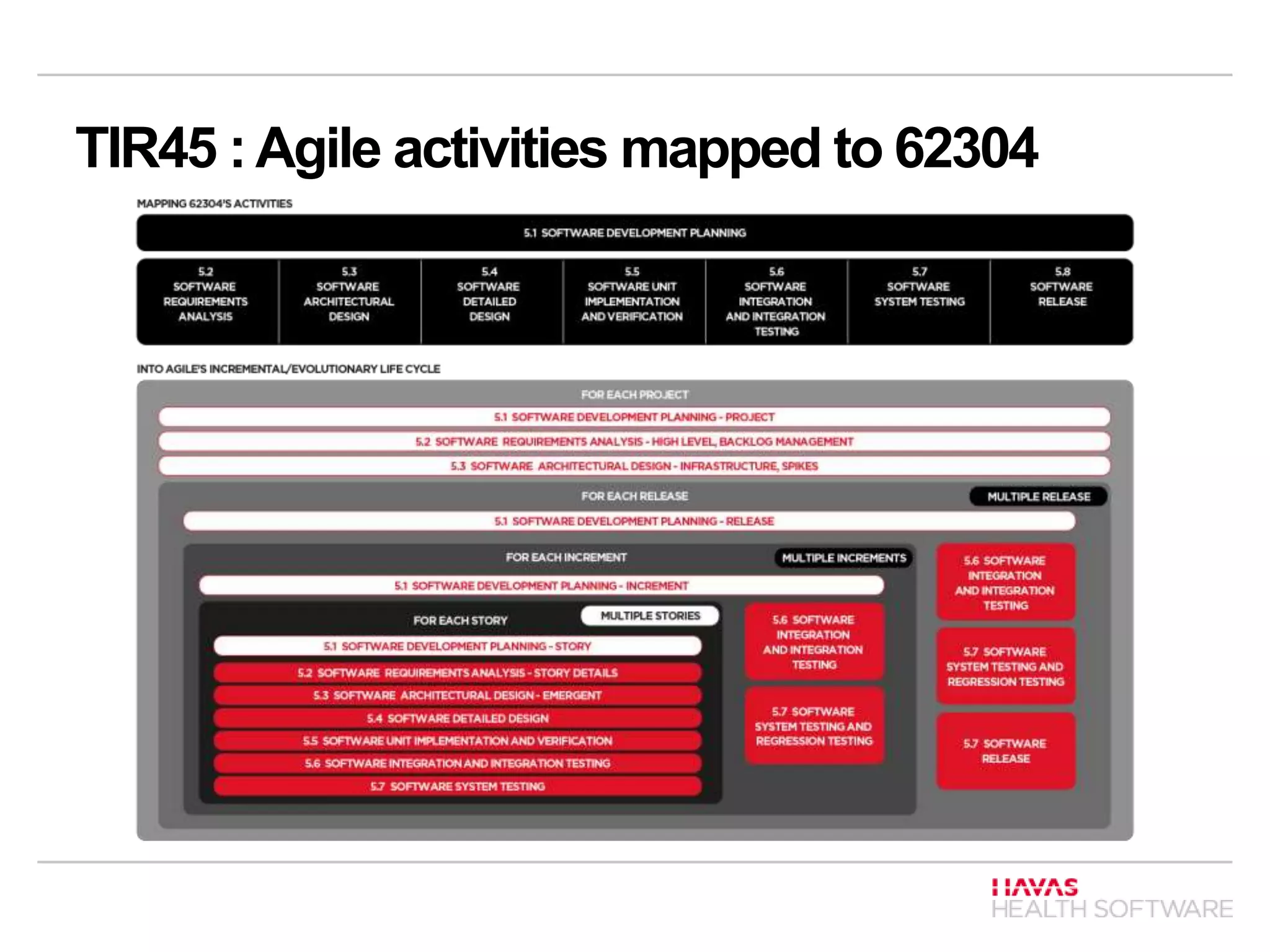
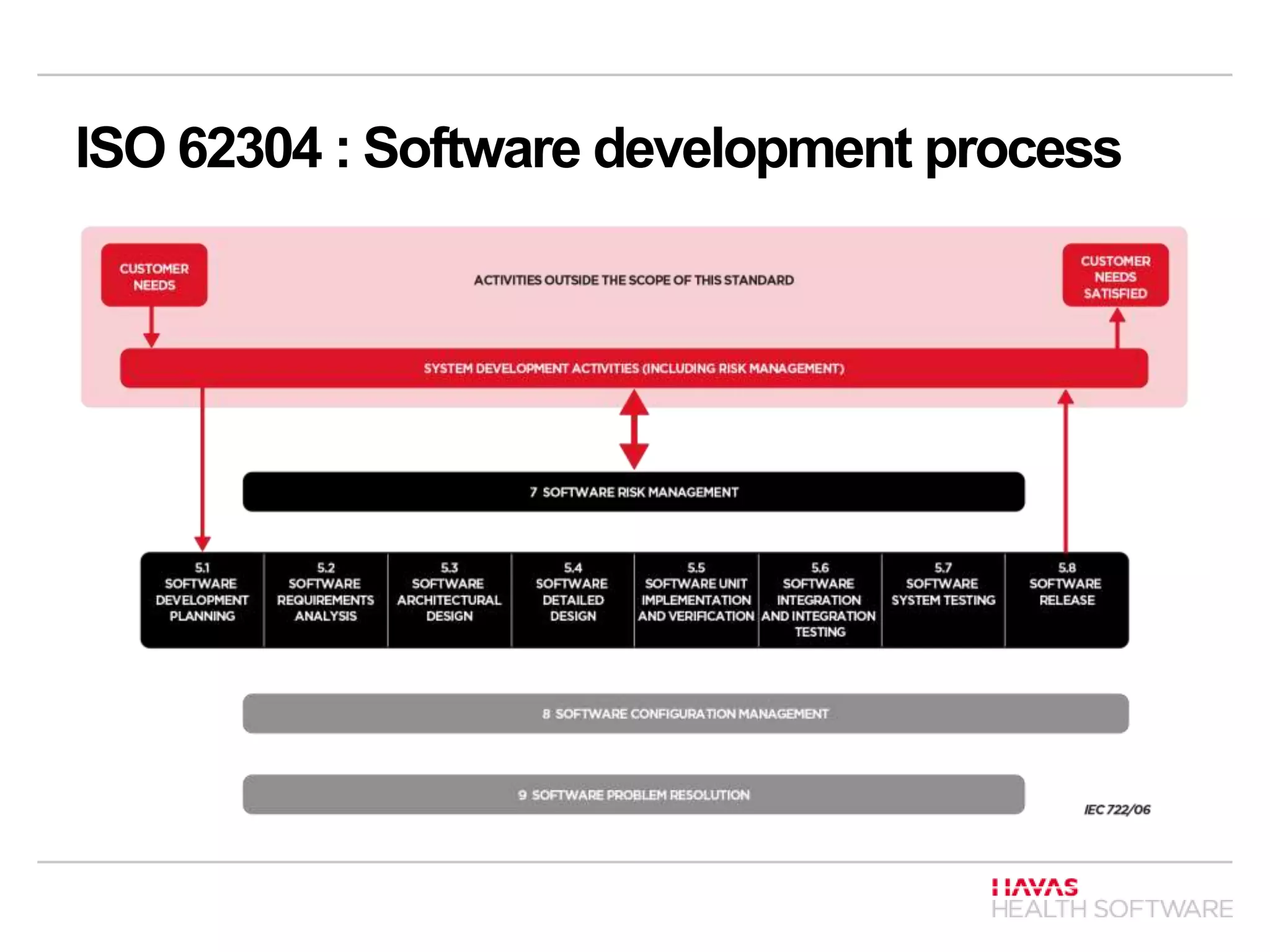
The document discusses ISO 62304 and TIR 45 in the context of software development for medical devices, underlining the requirements for risk and quality management processes. It outlines the software development lifecycle (SDLC) processes including software development, maintenance, risk management, and configuration management, emphasizing the classification of software systems based on potential harm. Additionally, it addresses the adaptability of ISO 62304 for Agile methods, mapping Agile principles to regulatory standards and emphasizing the importance of documentation and risk management.








![Software Development Plan [Class A, B, C]
− Processes, Methods, Tools
− Deliverables
− Functional Requirements
− Traceability between requirements and delivery
− software driven alarms/warnings/messages
− Security requirements
− UX requirements that sensitive to human error and training
− acceptance requirements
− What is the RISK PROCESS?
− What is the VERIFICATION PROCESS?](https://image.slidesharecdn.com/3827cehlshealth20marchpresentation-140804052957-phpapp01/75/ISO-62304-TIR-45-14-2048.jpg)
![Architecture and Design [Class B, C]
− Describes the software structure and identifies software items
− Describes the interfaces for software items
− Detailed designs for software items and interfaces
− Describes the system, functional and performance requirements
of SOUP software items
− RISK PROCESS
− Describe segregation between software items [Class C]
− VERIFICATION PROCESS](https://image.slidesharecdn.com/3827cehlshealth20marchpresentation-140804052957-phpapp01/75/ISO-62304-TIR-45-15-2048.jpg)
![Software Testing [Class B, C]
− Acceptance Plan/Process/Results
− Additional items required for Class C
− Unit Plan/Process/Results
− Integration Testing Plan/Process/Results
− Regression Plan/Process [Class A, B, C]
− RISK PROCESS
− VERIFICATION PROCESS](https://image.slidesharecdn.com/3827cehlshealth20marchpresentation-140804052957-phpapp01/75/ISO-62304-TIR-45-16-2048.jpg)
![Software Risk Process [Class B, C]
− Risk analysis for software
− Risk analysis for software changes
− Risk control measures
− VERIFICATION of risk control measures
− TRACEABILITY of risk controls
− Maintain a RISK MANGEMENT FILE](https://image.slidesharecdn.com/3827cehlshealth20marchpresentation-140804052957-phpapp01/75/ISO-62304-TIR-45-17-2048.jpg)
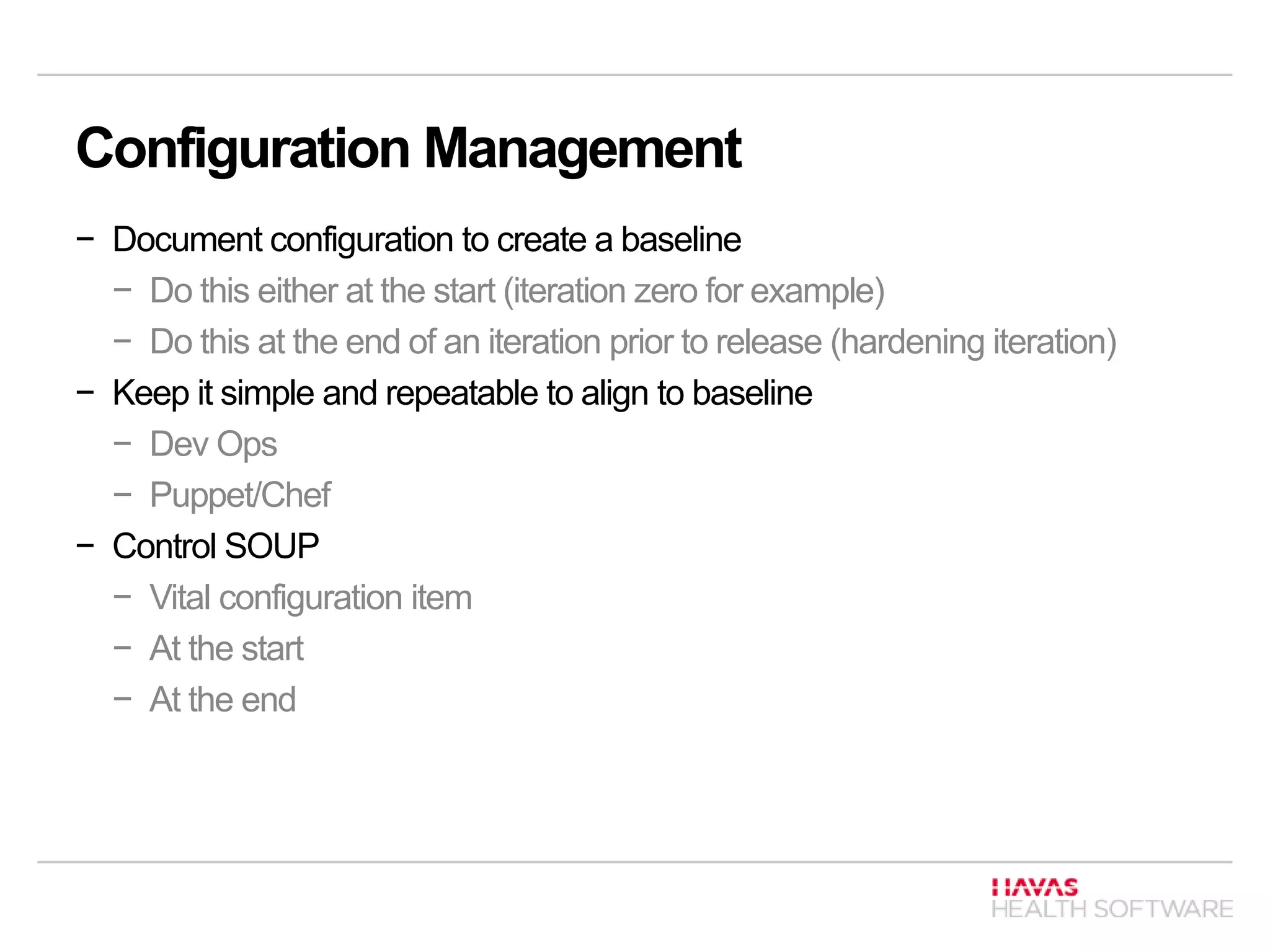
![Configuration Management [Class A, B, C]
Identify configuration items
− Software
− Hardware
Identify SOUP configuration items
− Both external and internal items
Document configuration items
− SOP how the items are configured, by who, when etc.](https://image.slidesharecdn.com/3827cehlshealth20marchpresentation-140804052957-phpapp01/75/ISO-62304-TIR-45-18-2048.jpg)
![Change Management [Class A, B, C]
− Records of change requests
− Change requests have to be approved prior to implementation
− Cross check software classification as a result of change
− VERIFICATION of change
− TRACEABILITY of change](https://image.slidesharecdn.com/3827cehlshealth20marchpresentation-140804052957-phpapp01/75/ISO-62304-TIR-45-19-2048.jpg)
![Software problem resolution [Class A, B, C]
− Prepare problem reports
− type, scope and critically
− Investigate the problem
− Advise relevant parties
− Use change control process
− Maintain records of problems, resolutions and VERIFICATION of resolution
− Update RISK MANGEMENT FILE if required](https://image.slidesharecdn.com/3827cehlshealth20marchpresentation-140804052957-phpapp01/75/ISO-62304-TIR-45-20-2048.jpg)