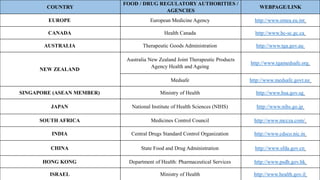
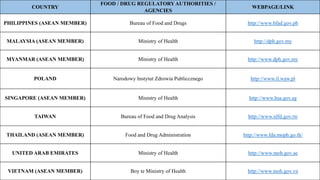
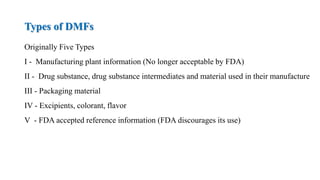
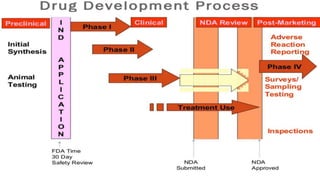
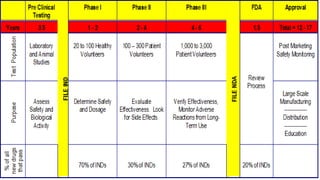
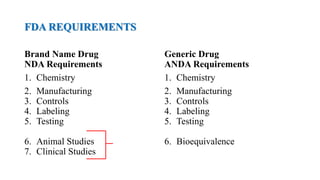
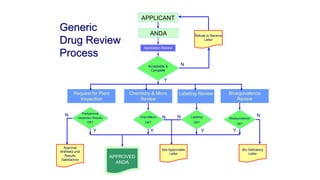
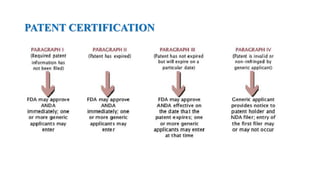
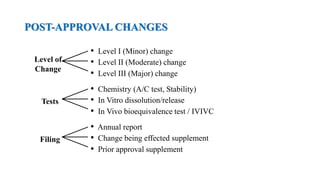
The document discusses regulations for drug submissions and applications in the US and other countries. It covers the major regulatory bodies like the FDA and EMA. It then focuses on the different types of applications filed with the FDA - Drug Master Files (DMF), Investigational New Drug Application (IND), New Drug Application (NDA), and Abbreviated New Drug Application (ANDA). The ANDA process for generic drugs is summarized, including requirements for bioequivalence testing and patent certification in the Orange Book.