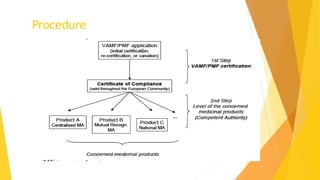
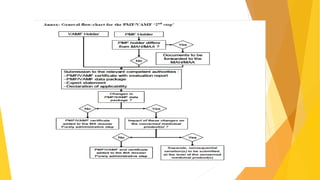
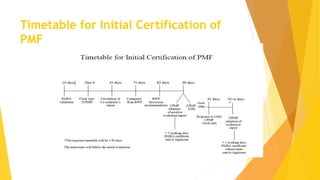
The Plasma Master File (PMF) is a comprehensive documentation essential for ensuring the quality and safety of human plasma used in medical products and is distinct from marketing authorization dossiers. Submitted and updated annually, its certification involves a structured evaluation process with defined timetables by the European Medicines Agency. The PMF submission requires specific documentation organized within the eCTD structure, including a cover letter, application form, and compliance certificate.