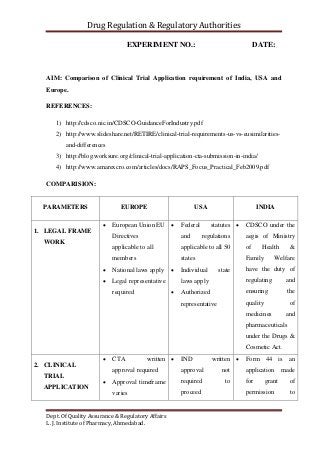
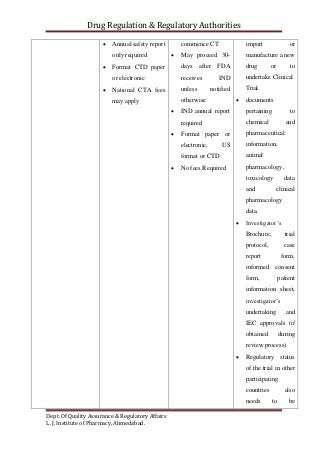
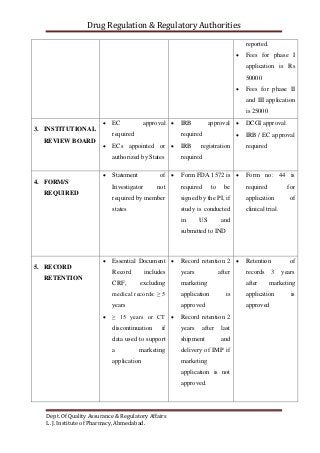
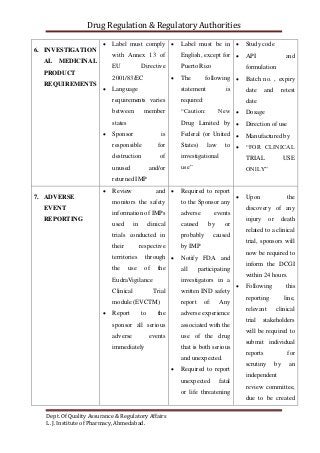
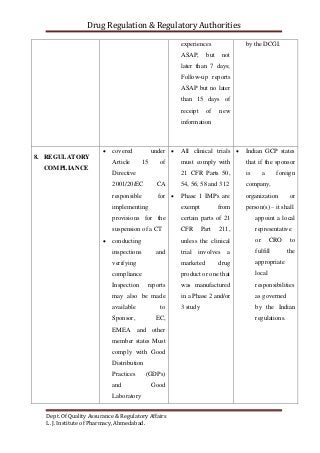
The document compares the clinical trial application requirements of India, the United States, and Europe. Some key differences include:
- Europe requires approval of a clinical trial application, while the US only requires an investigational new drug application be filed.
- India requires forms, documentation of chemical/toxicology data, and fees to be submitted with the application.
- The US, Europe, and India all require institutional review board or ethics committee approval before starting a trial.
- Reporting and retention of adverse events and trial records differs between the regions' regulations.