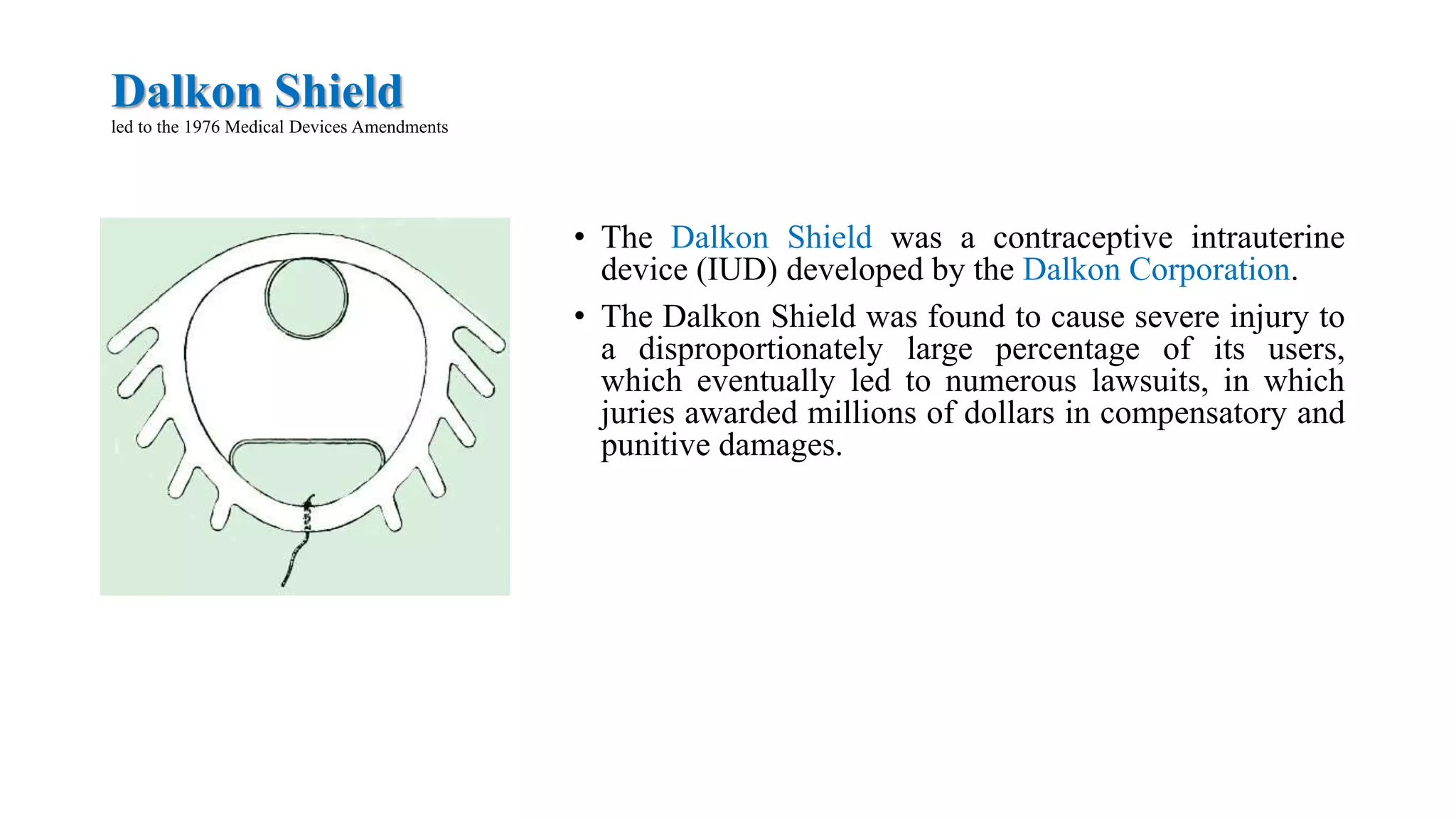
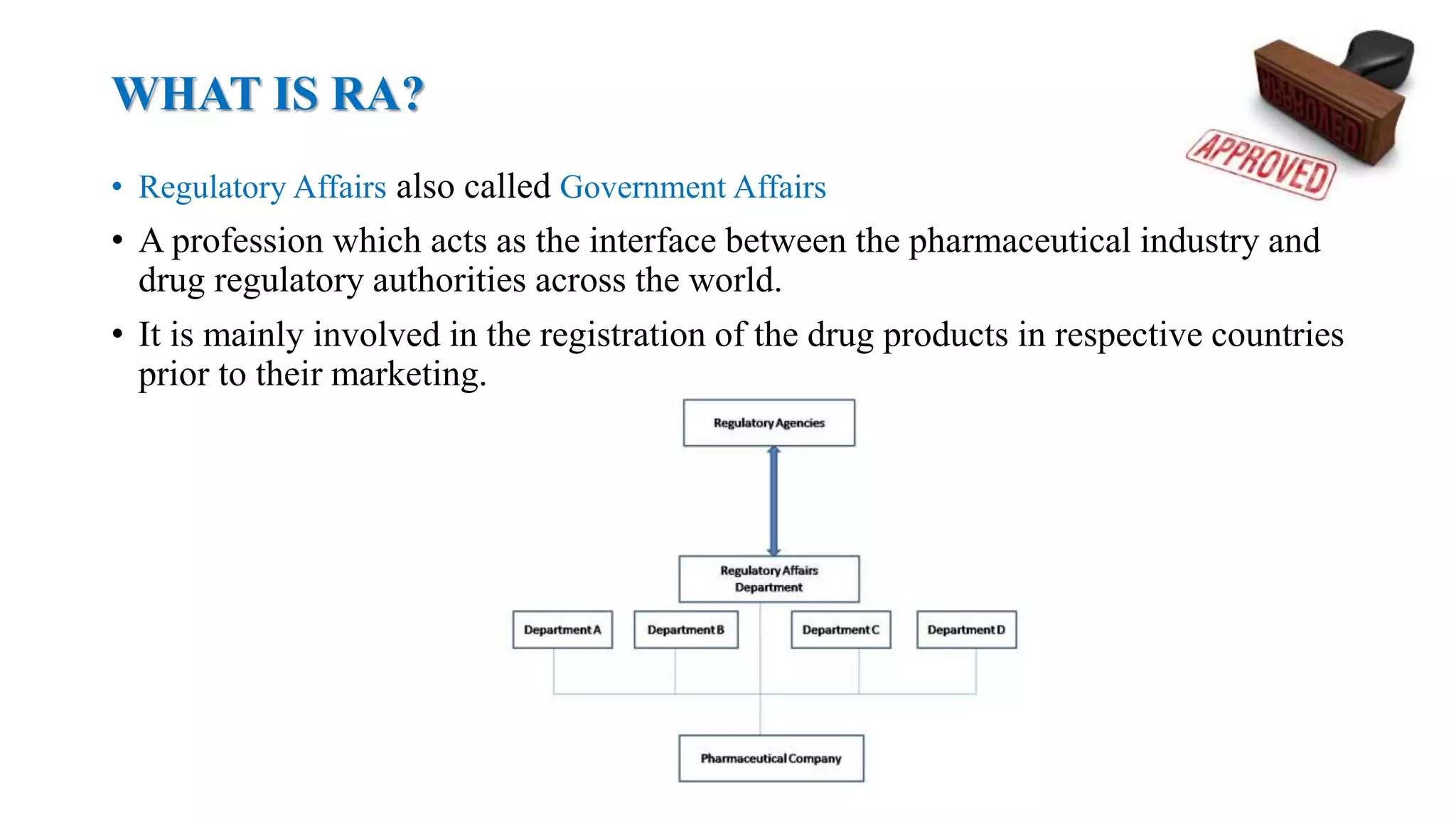
Regulatory affairs emerged from disasters in the pharmaceutical industry that caused increased legislation around drug safety and quality. Key events that shaped regulations include contaminated vaccines that killed children, unsanitary meat production exposed in The Jungle, an improperly prepared sulfanilamide medicine that killed over 100 people, the thalidomide morning sickness drug that caused birth defects, problematic intrauterine devices like the Dalkon Shield, and mechanical heart valves like the Bjork-Shiley that malfunctioned. Regulatory affairs acts as the interface between industry and drug authorities, ensuring the registration, safety, efficacy and quality of pharmaceutical products according to legislative requirements in different countries.