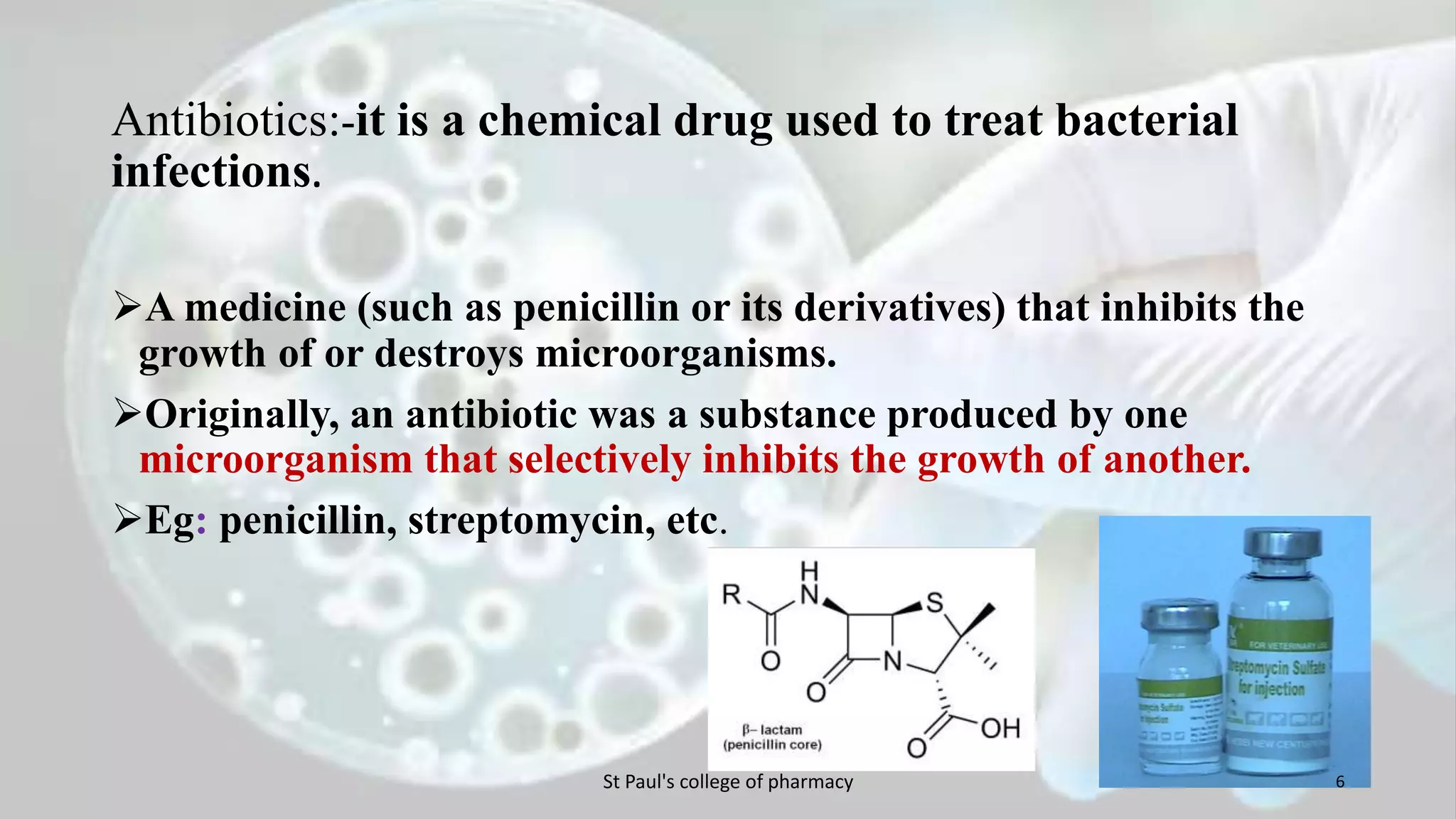
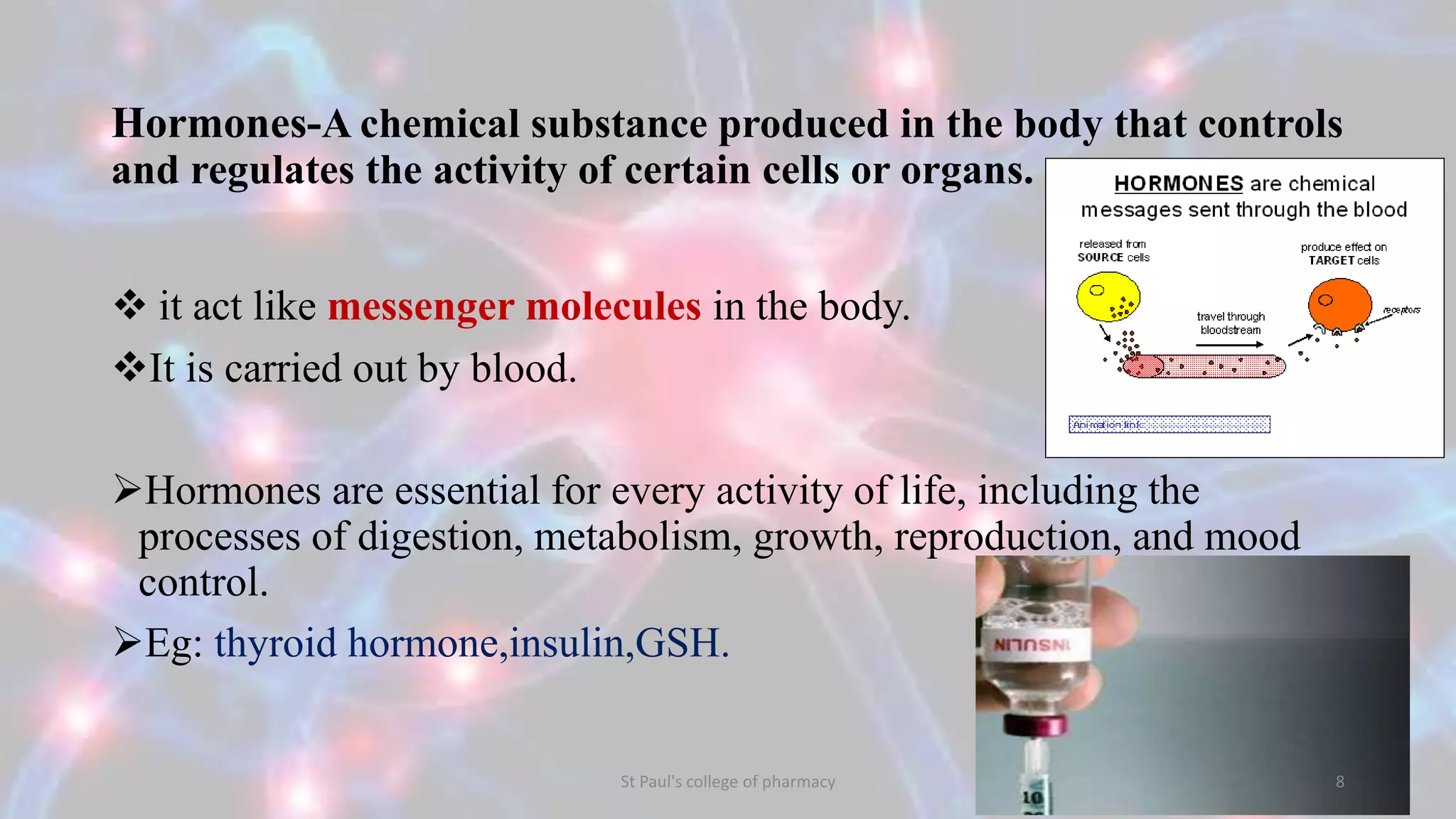
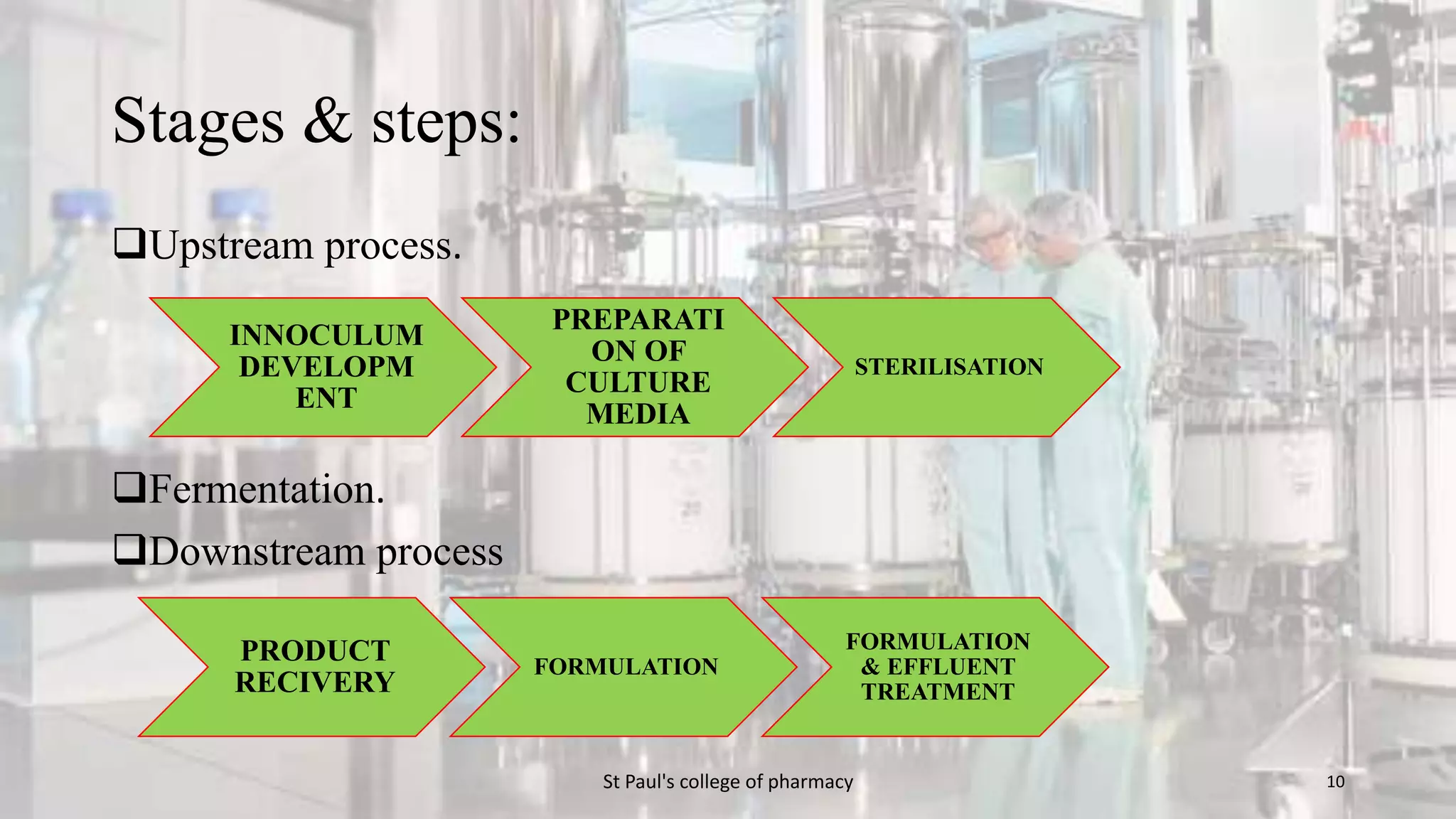
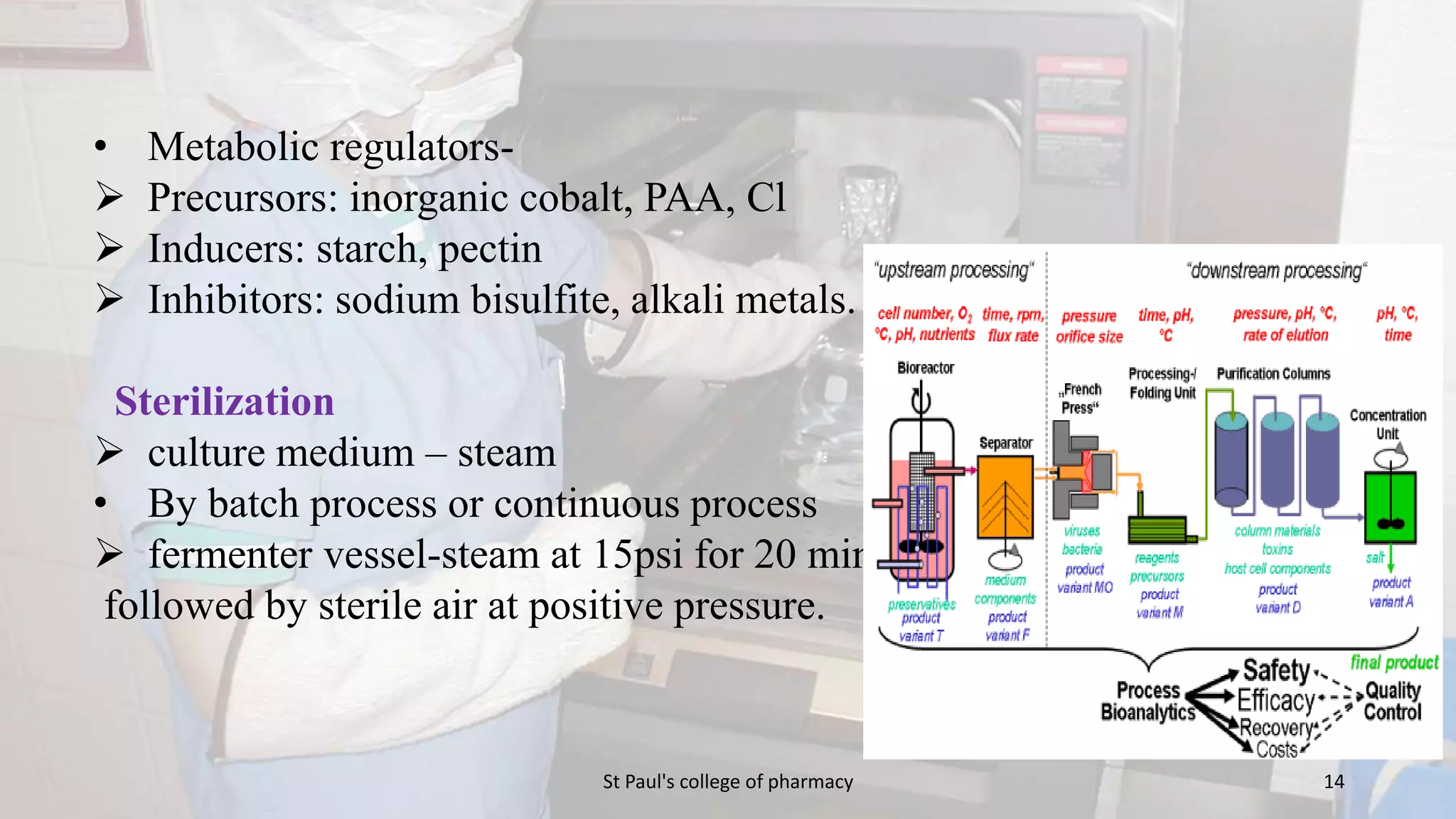
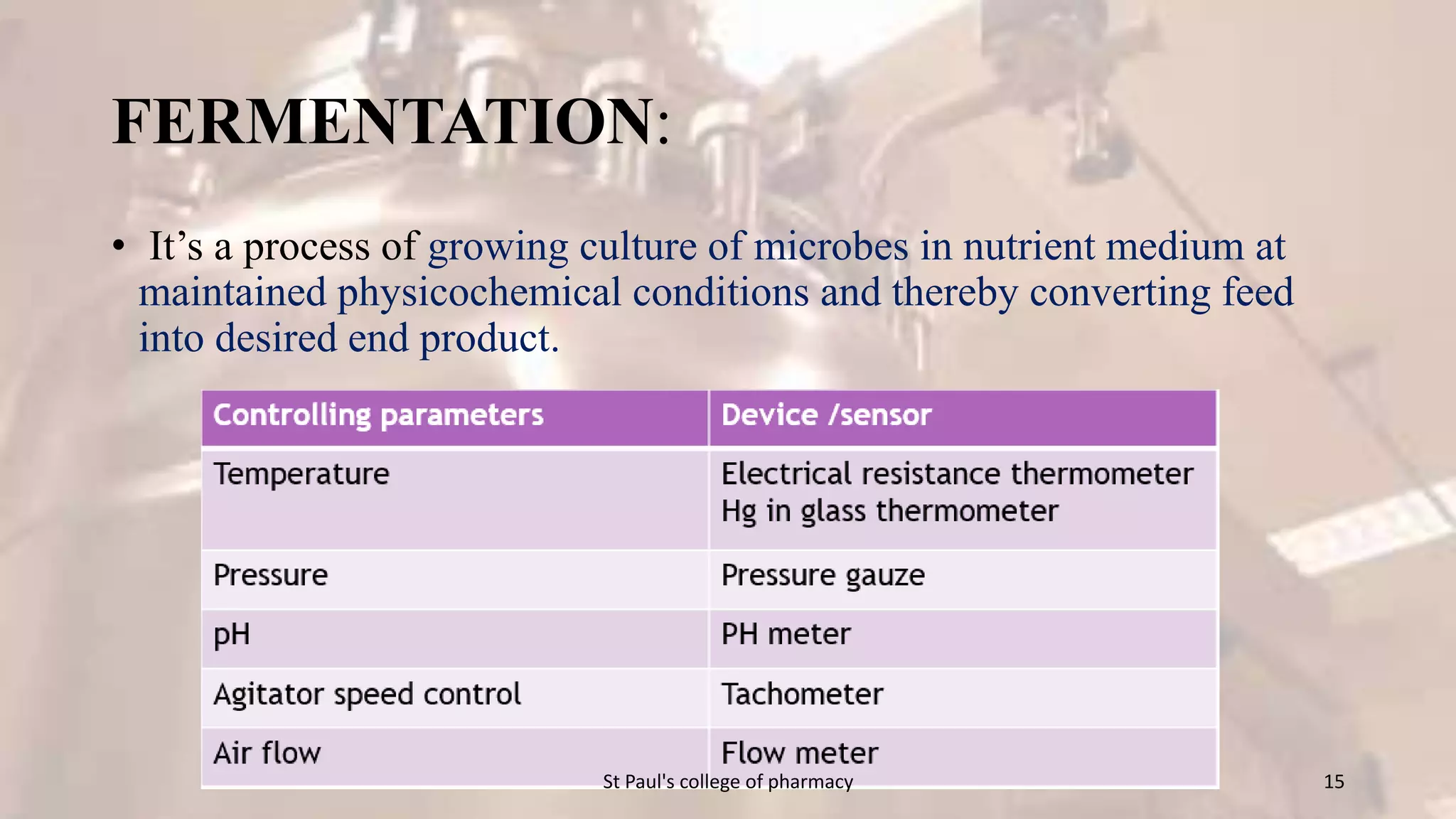
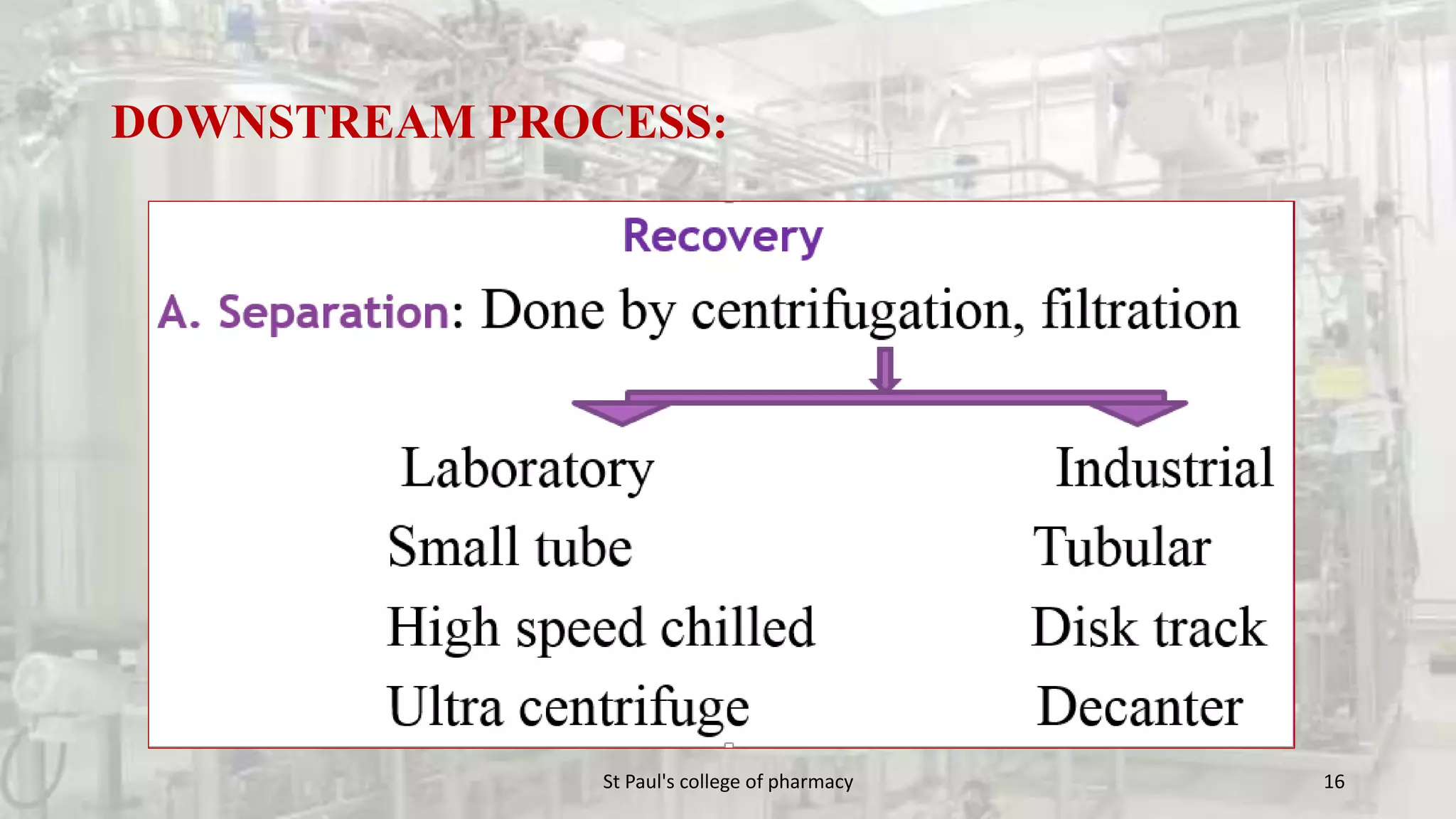
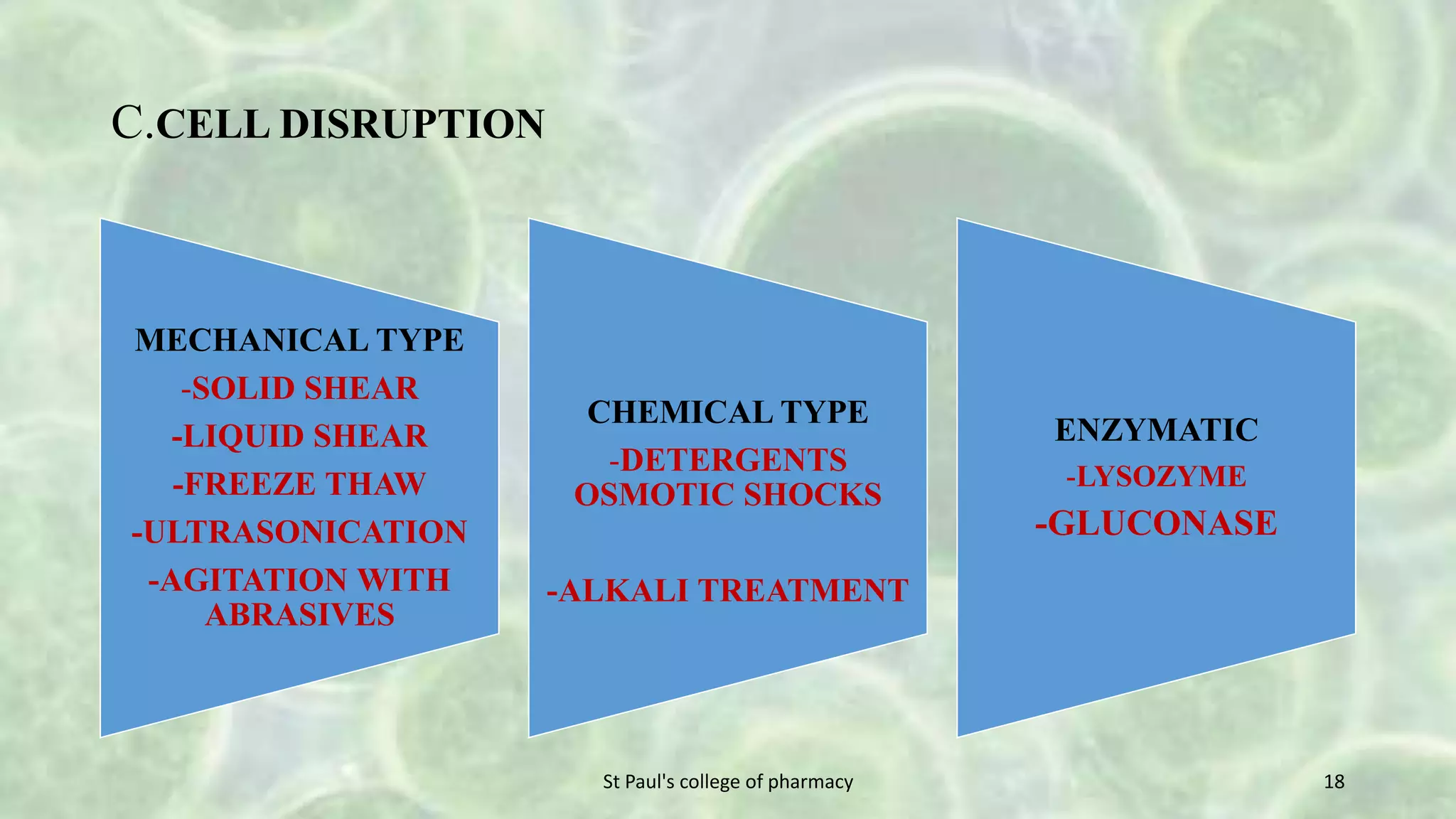
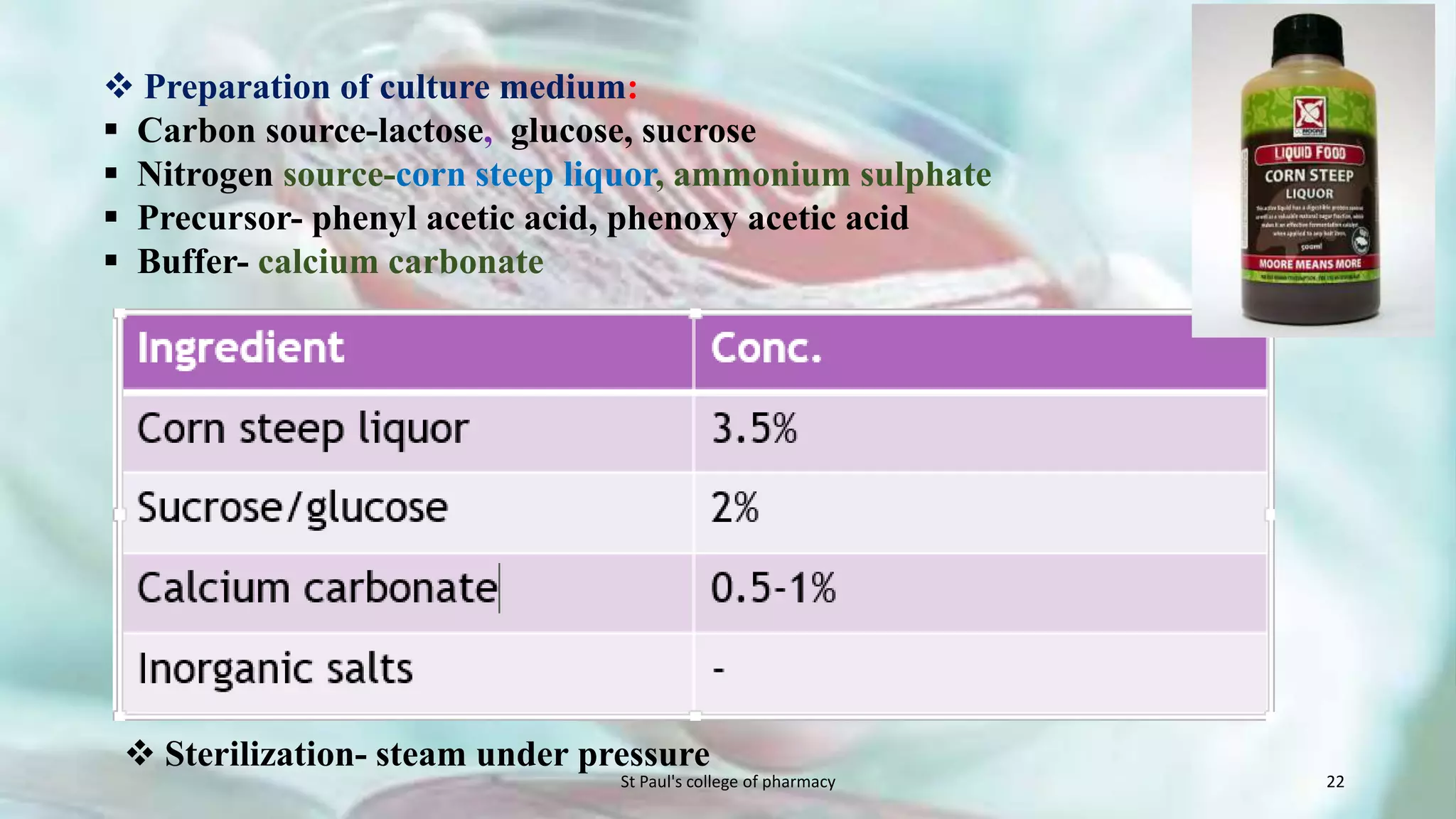
The document discusses upstream and downstream processing of antibiotics, hormones, and vaccines. Upstream processing involves fermentation and includes inoculum preparation, culture media development, and fermentation. Downstream processing refers to product recovery, purification, and formulation stages after fermentation. These include steps like separation, concentration, purification. The document provides details of these processes for antibiotics like penicillin, hormones, and vaccines.