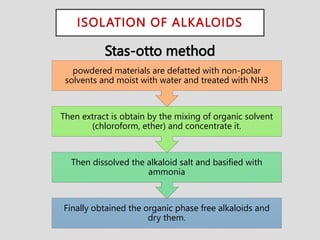
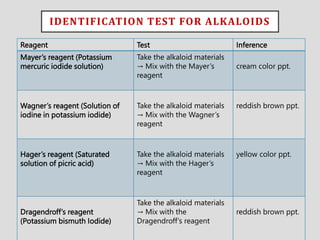
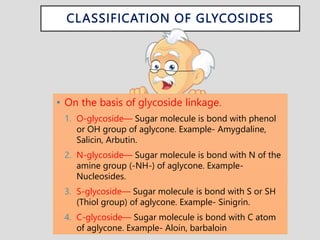
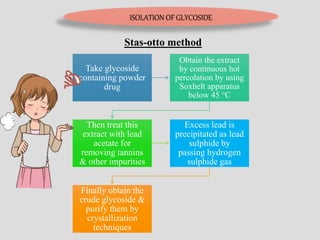
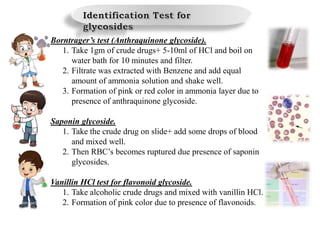
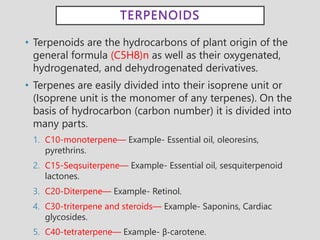
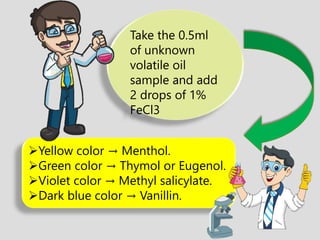
The document provides an overview of alkaloids, glycosides, tannins, volatile oils, terpenoids, and resins, detailing their definitions, classifications, isolation methods, identification tests, and therapeutic applications. Alkaloids include basic nitrogenous compounds with various subtypes like true alkaloids and pseudo alkaloids, while glycosides consist of sugar moieties linked to aglycones. Tannins, volatile oils, and terpenoids are presented with their astringent properties, flavoring applications, and medicinal uses, highlighting their significance in pharmacy.