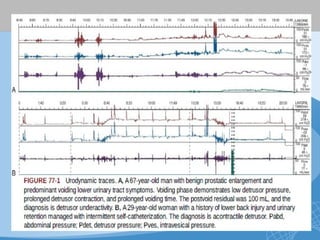
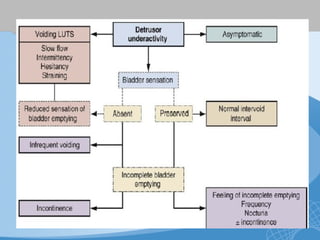
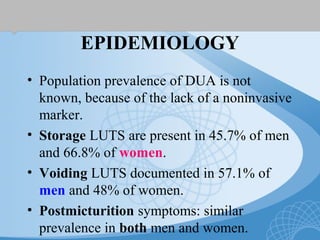
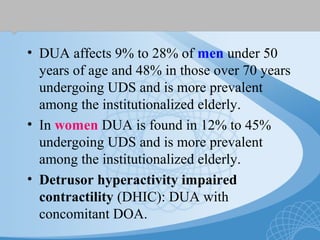
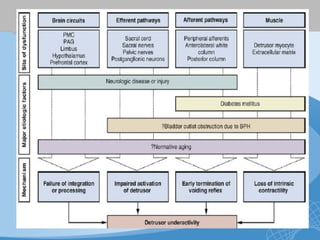
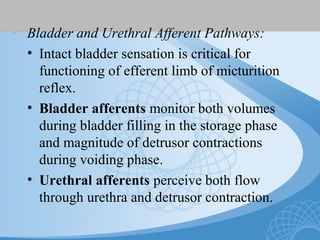
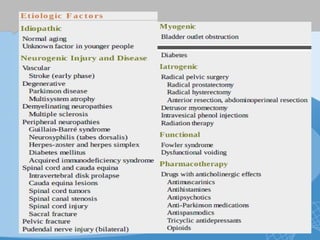
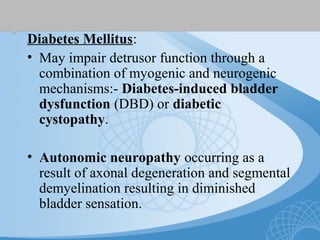
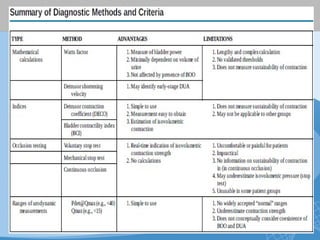
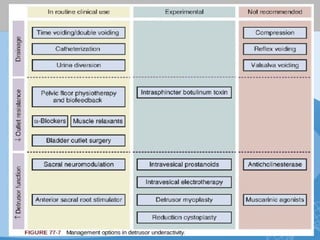
This document discusses underactive detrusor, defined as a contraction of reduced strength and/or duration that results in prolonged bladder emptying or failure to empty. It may be caused by myogenic factors like detrusor muscle dysfunction or neurogenic factors affecting bladder nerves. Symptoms include hesitancy, weak stream, and incomplete emptying. Diagnosis is by urodynamics, though no universal criteria exist. Management focuses on bladder drainage via catheters or relaxing the bladder outlet to improve emptying, as no treatments directly improve detrusor function. Options include scheduled voiding, pelvic floor therapy, catheters, drugs, electrical stimulation, botulinum toxin, and rarely surgery.